Stoichiometry Molar relationships in chemical reactions 3 1








- Slides: 36

Stoichiometry Molar relationships in chemical reactions

3. 1 Chemical Equations: A Review • Law of conservation of mass • Relationship between reactant and products produces a balanced chemical equation • Reactants Products A+B C+D • Balancing chemical eqns

Balancing Chemical Equations • Must have correct chemical formulas • Change coefficients as needed to balance equation • Do not change subscripts of chemical formulas • Indicate physical states by writing in parentheses (s), (l), (g), (aq) • Begin with an element that appears in only one reactant and product • If possible, do not begin with O or H

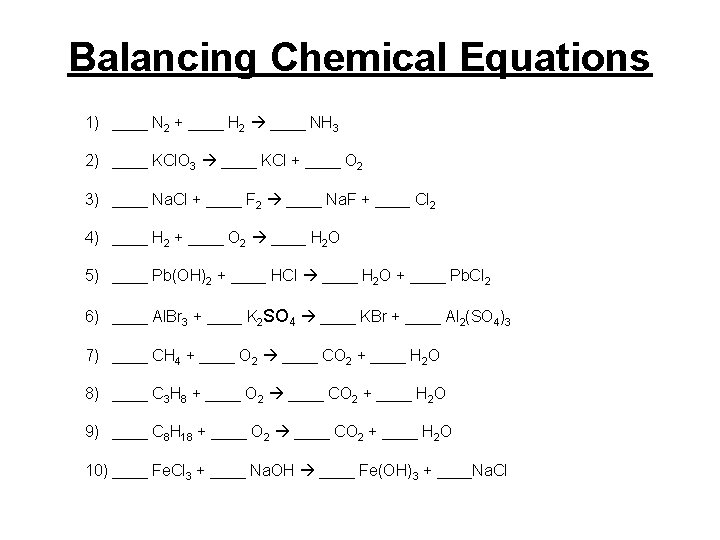
Balancing Chemical Equations 1) ____ N 2 + ____ H 2 ____ NH 3 2) ____ KCl. O 3 ____ KCl + ____ O 2 3) ____ Na. Cl + ____ F 2 ____ Na. F + ____ Cl 2 4) ____ H 2 + ____ O 2 ____ H 2 O 5) ____ Pb(OH)2 + ____ HCl ____ H 2 O + ____ Pb. Cl 2 6) ____ Al. Br 3 + ____ K 2 SO 4 ____ KBr + ____ Al 2(SO 4)3 7) ____ CH 4 + ____ O 2 ____ CO 2 + ____ H 2 O 8) ____ C 3 H 8 + ____ O 2 ____ CO 2 + ____ H 2 O 9) ____ C 8 H 18 + ____ O 2 ____ CO 2 + ____ H 2 O 10) ____ Fe. Cl 3 + ____ Na. OH ____ Fe(OH)3 + ____Na. Cl

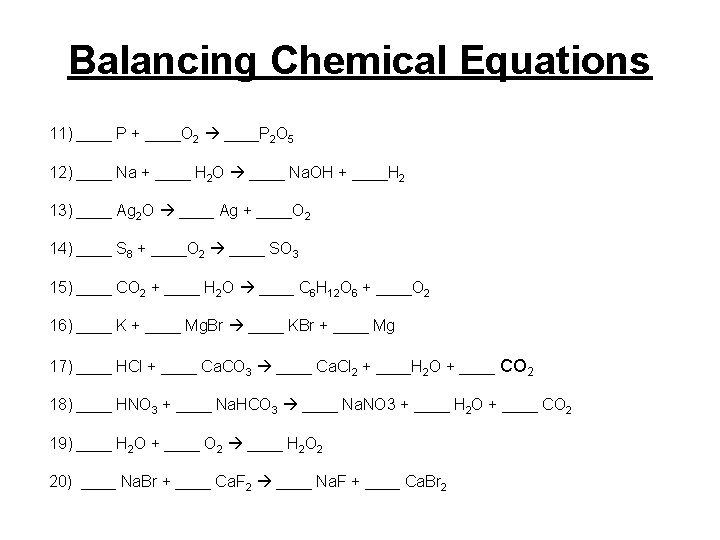
Balancing Chemical Equations 11) ____ P + ____O 2 ____P 2 O 5 12) ____ Na + ____ H 2 O ____ Na. OH + ____H 2 13) ____ Ag 2 O ____ Ag + ____O 2 14) ____ S 8 + ____O 2 ____ SO 3 15) ____ CO 2 + ____ H 2 O ____ C 6 H 12 O 6 + ____O 2 16) ____ K + ____ Mg. Br ____ KBr + ____ Mg 17) ____ HCl + ____ Ca. CO 3 ____ Ca. Cl 2 + ____H 2 O + ____ CO 2 18) ____ HNO 3 + ____ Na. HCO 3 ____ Na. NO 3 + ____ H 2 O + ____ CO 2 19) ____ H 2 O + ____ O 2 ____ H 2 O 2 20) ____ Na. Br + ____ Ca. F 2 ____ Na. F + ____ Ca. Br 2

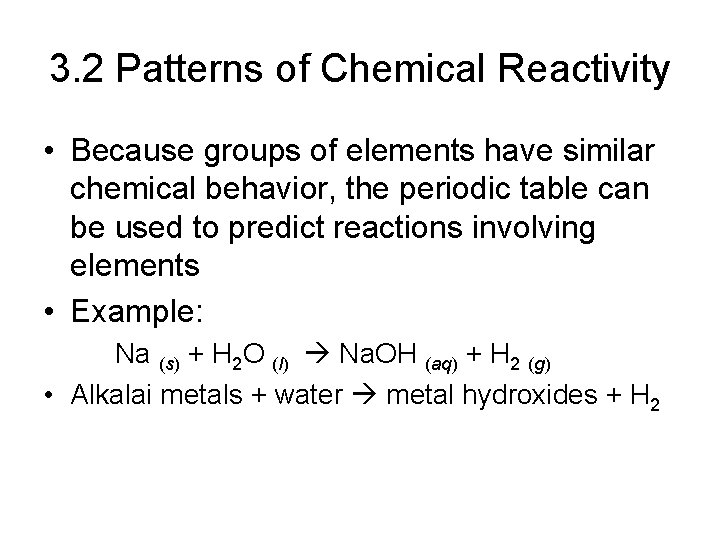
3. 2 Patterns of Chemical Reactivity • Because groups of elements have similar chemical behavior, the periodic table can be used to predict reactions involving elements • Example: Na (s) + H 2 O (l) Na. OH (aq) + H 2 (g) • Alkalai metals + water metal hydroxides + H 2

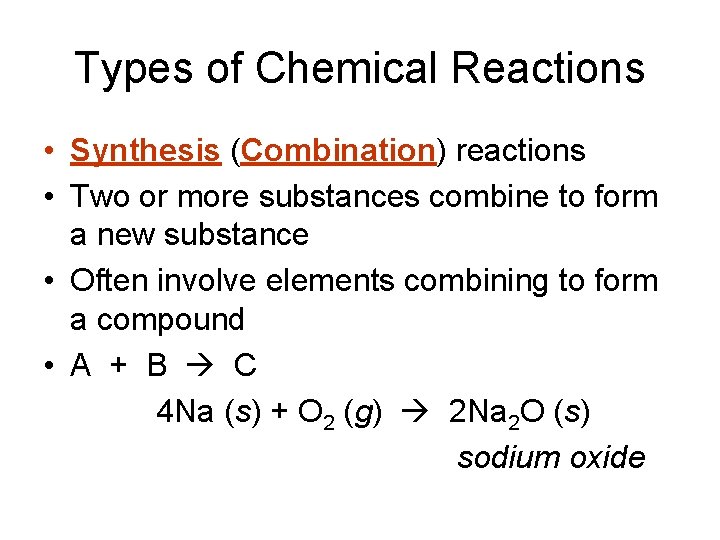
Types of Chemical Reactions • Synthesis (Combination) reactions • Two or more substances combine to form a new substance • Often involve elements combining to form a compound • A + B C 4 Na (s) + O 2 (g) 2 Na 2 O (s) sodium oxide

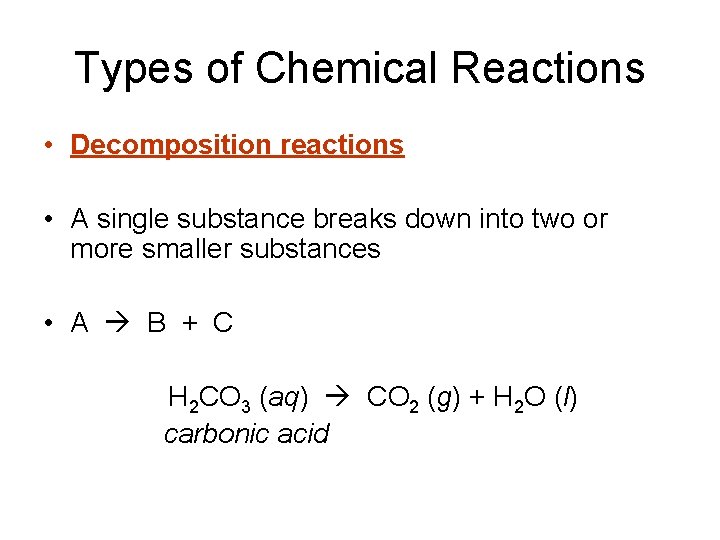
Types of Chemical Reactions • Decomposition reactions • A single substance breaks down into two or more smaller substances • A B + C H 2 CO 3 (aq) CO 2 (g) + H 2 O (l) carbonic acid

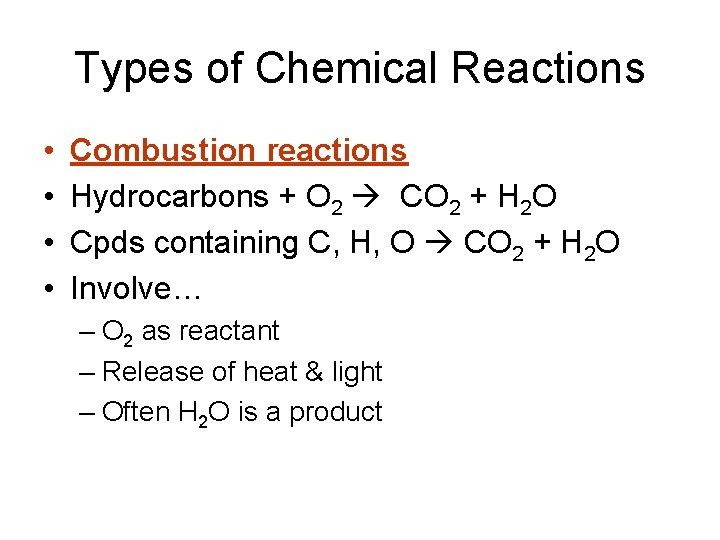
Types of Chemical Reactions • • Combustion reactions Hydrocarbons + O 2 CO 2 + H 2 O Cpds containing C, H, O CO 2 + H 2 O Involve… – O 2 as reactant – Release of heat & light – Often H 2 O is a product

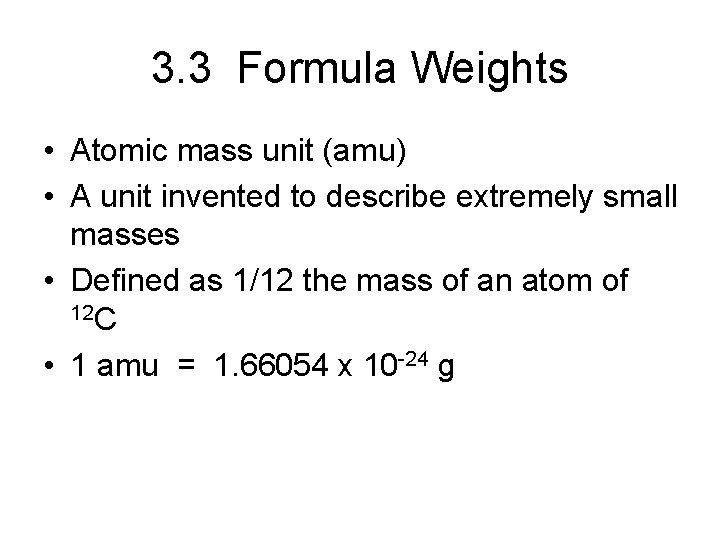
3. 3 Formula Weights • Atomic mass unit (amu) • A unit invented to describe extremely small masses • Defined as 1/12 the mass of an atom of 12 C • 1 amu = 1. 66054 x 10 -24 g

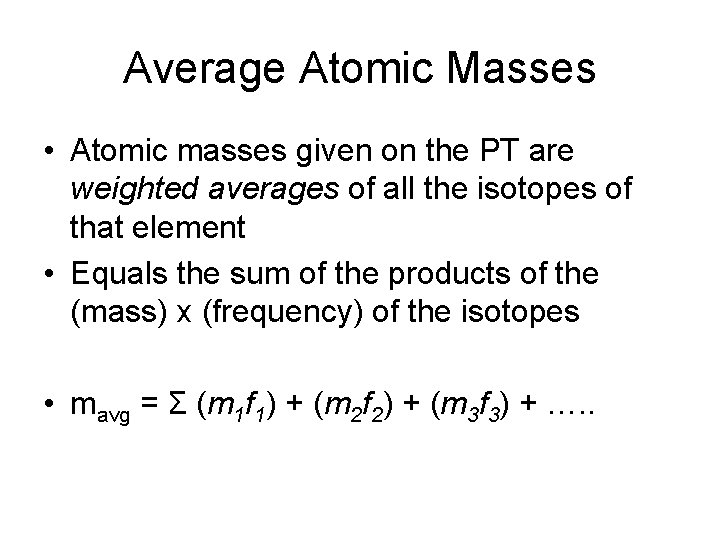
Average Atomic Masses • Atomic masses given on the PT are weighted averages of all the isotopes of that element • Equals the sum of the products of the (mass) x (frequency) of the isotopes • mavg = Σ (m 1 f 1) + (m 2 f 2) + (m 3 f 3) + …. .

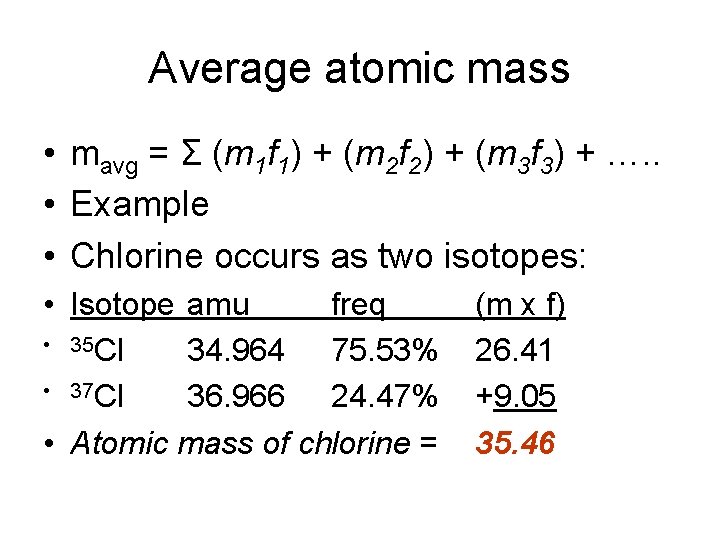
Average atomic mass • mavg = Σ (m 1 f 1) + (m 2 f 2) + (m 3 f 3) + …. . • Example • Chlorine occurs as two isotopes: • Isotope amu freq • 35 Cl 34. 964 75. 53% • 37 Cl 36. 966 24. 47% • Atomic mass of chlorine = (m x f) 26. 41 +9. 05 35. 46

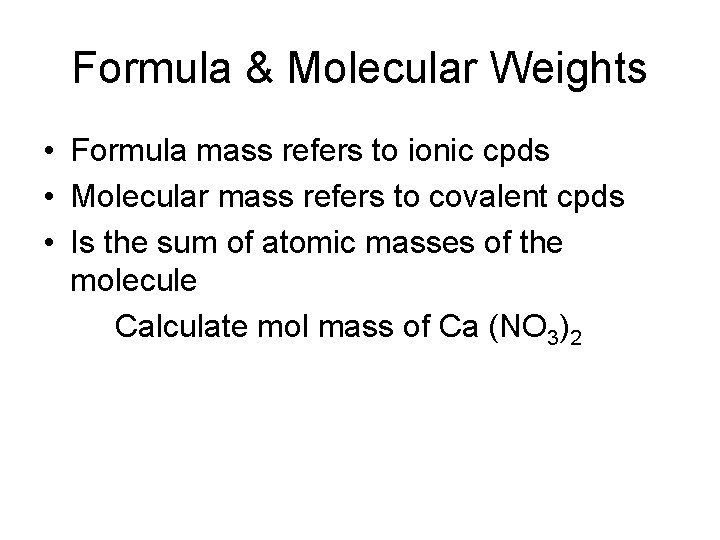
Formula & Molecular Weights • Formula mass refers to ionic cpds • Molecular mass refers to covalent cpds • Is the sum of atomic masses of the molecule Calculate mol mass of Ca (NO 3)2

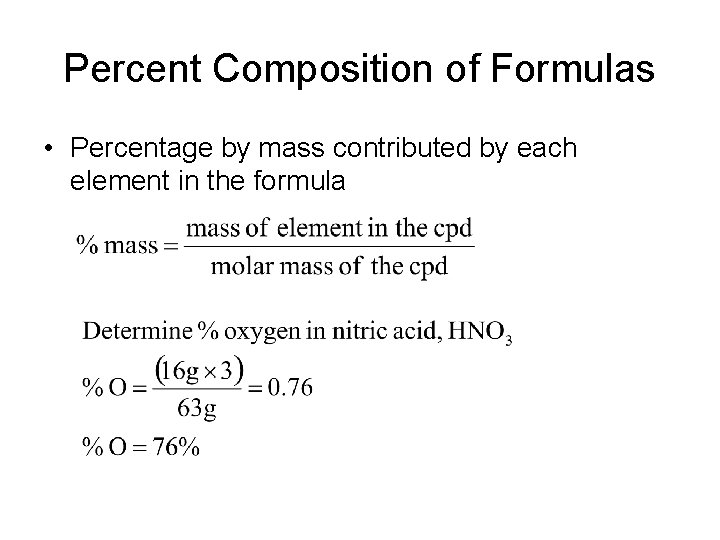
Percent Composition of Formulas • Percentage by mass contributed by each element in the formula

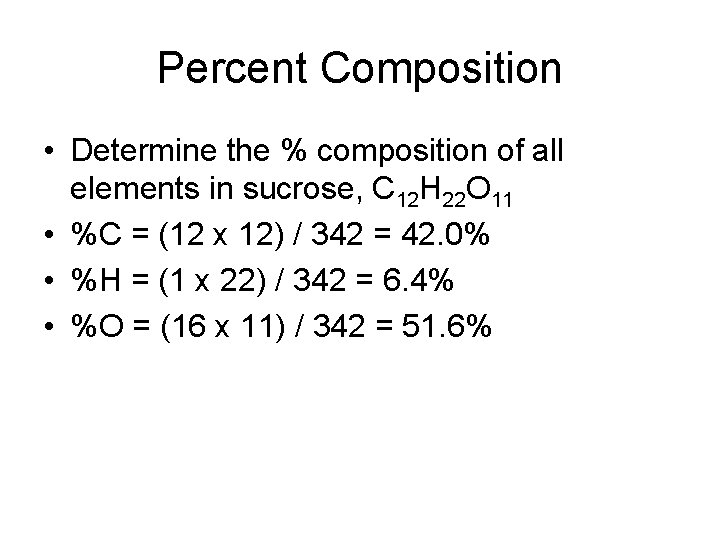
Percent Composition • Determine the % composition of all elements in sucrose, C 12 H 22 O 11 • %C = (12 x 12) / 342 = 42. 0% • %H = (1 x 22) / 342 = 6. 4% • %O = (16 x 11) / 342 = 51. 6%

3. 4 The Mole • • Is a counting unit Avagadro’s number = 6. 022 x 1023 Interconvert between moles & particles Molar mass = the mass of one mole of a substance • Molar mass of elements = atomic mass in grams

Converting between particles, moles, and grams • Particles and grams cannot be interconverted directly. • The mole is the bridge between particles and grams.

3. 5 Empirical Formula Analysis • To determine empirical formula from percent composition data • Empirical formula gives simplest ratio of atoms in a compound • Therefore, always convert to moles when calculating empirical formulas • Sample exercise 3. 13

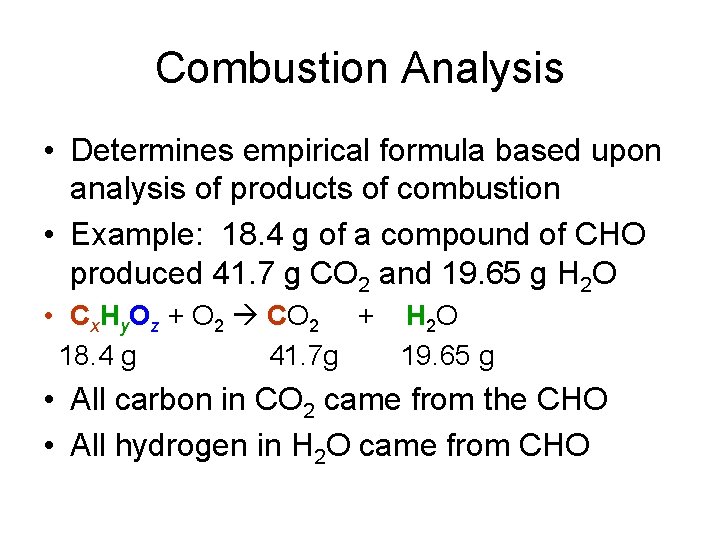
Combustion Analysis • Determines empirical formula based upon analysis of products of combustion • Example: 18. 4 g of a compound of CHO produced 41. 7 g CO 2 and 19. 65 g H 2 O • Cx. Hy. Oz + O 2 CO 2 + H 2 O 18. 4 g 41. 7 g 19. 65 g • All carbon in CO 2 came from the CHO • All hydrogen in H 2 O came from CHO

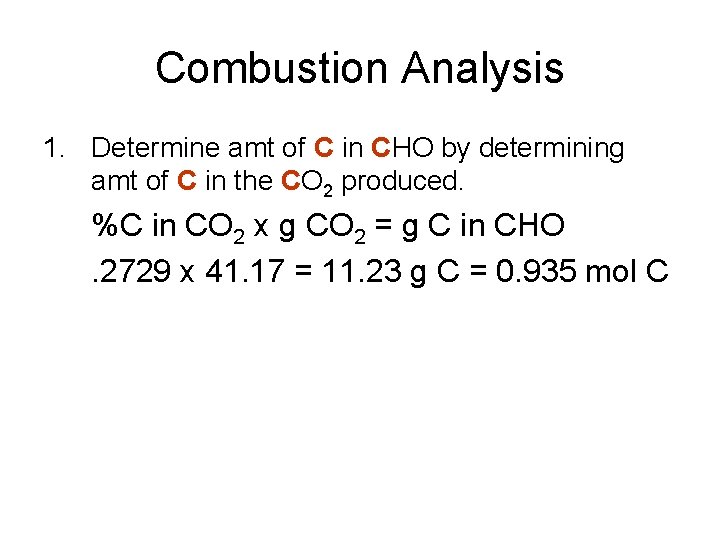
Combustion Analysis 1. Determine amt of C in CHO by determining amt of C in the CO 2 produced. %C in CO 2 x g CO 2 = g C in CHO. 2729 x 41. 17 = 11. 23 g C = 0. 935 mol C

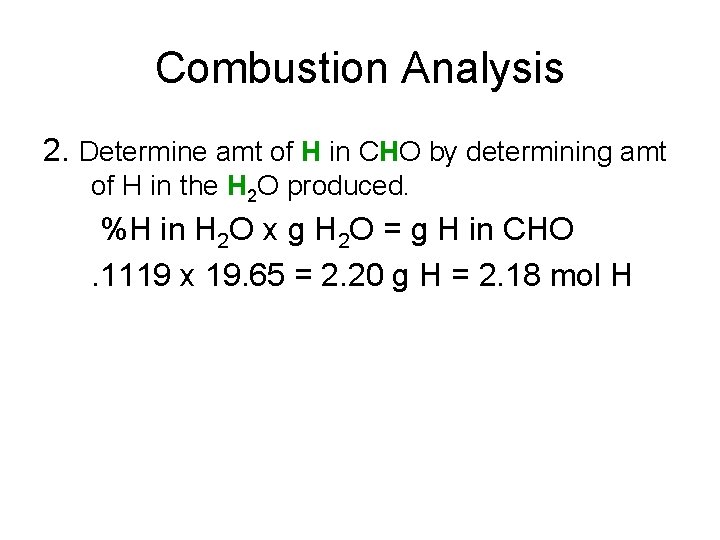
Combustion Analysis 2. Determine amt of H in CHO by determining amt of H in the H 2 O produced. %H in H 2 O x g H 2 O = g H in CHO. 1119 x 19. 65 = 2. 20 g H = 2. 18 mol H

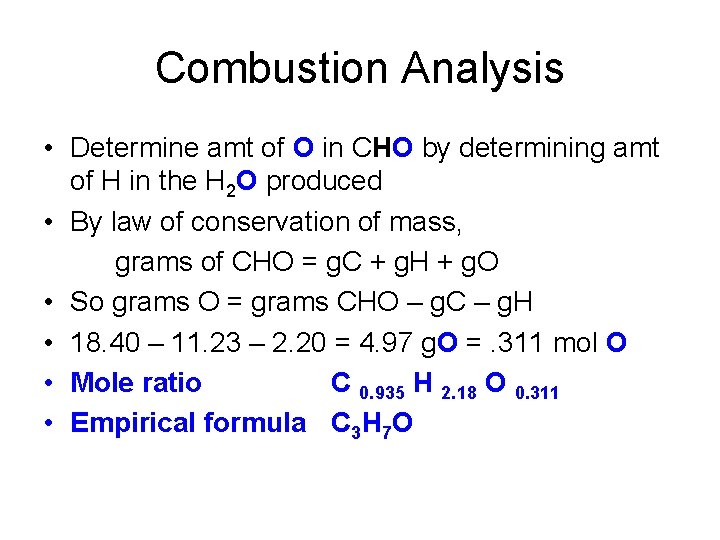
Combustion Analysis • Determine amt of O in CHO by determining amt of H in the H 2 O produced • By law of conservation of mass, grams of CHO = g. C + g. H + g. O • So grams O = grams CHO – g. C – g. H • 18. 40 – 11. 23 – 2. 20 = 4. 97 g. O =. 311 mol O • Mole ratio C 0. 935 H 2. 18 O 0. 311 • Empirical formula C 3 H 7 O

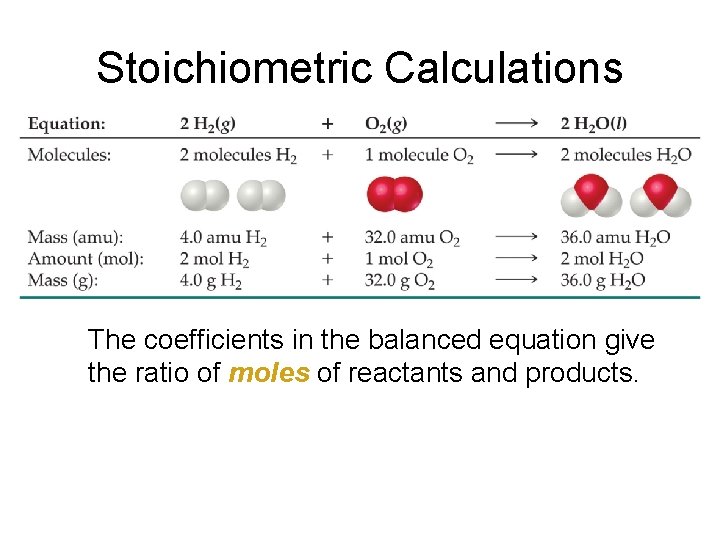
3. 6 Quantitative Information from Balanced Equations • Balanced chemical equations are like specific recipes • Balanced equations tell us …. – the relative amounts of reactants (ingredients) – the relative amounts of products – other relevant conditions needed (e. g. heat) • The unit of measure in a balanced equation is the mole – Indicated by coefficients

Apple Snapple Oatmeal (www. Mr. Breakfast. com) Apple Snapple Oatmeal (4 servings) 1 medium apple or pear 3 cups apple juice or cider 1 and 1/3 cups regular rolled oats 1/4 cup raisins or chopped pitted dates 1/4 teaspoon ground cinnamon milk (if you like) brown sugar (if you like)

Stoichiometric Calculations The coefficients in the balanced equation give the ratio of moles of reactants and products.

3. 6 Quantitative Information from Balanced Equations • Mole ratio used to calculate actual molar amounts in a balanced eqn • Sample Problem 3. 16 • Determine mass of water produced from combustion of 1. 00 g glucose • C 6 H 12 O 6 + __ O 2 __ CO 2 +__ H 2 O • C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O

Solving Stoichiometry Problems

Sample problem • Determine mass of water produced from combustion of 1. 00 g glucose • C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O • Determine moles glucose • Determine moles water • Determine grams water

3. 7 Limiting Reactants • Stoichiometrically least abundant reactant • Completely consumed in the reaction • Determines theoretical yield of the reaction • When all reactants are present in stoichiometric amounts, all are limiting reagents

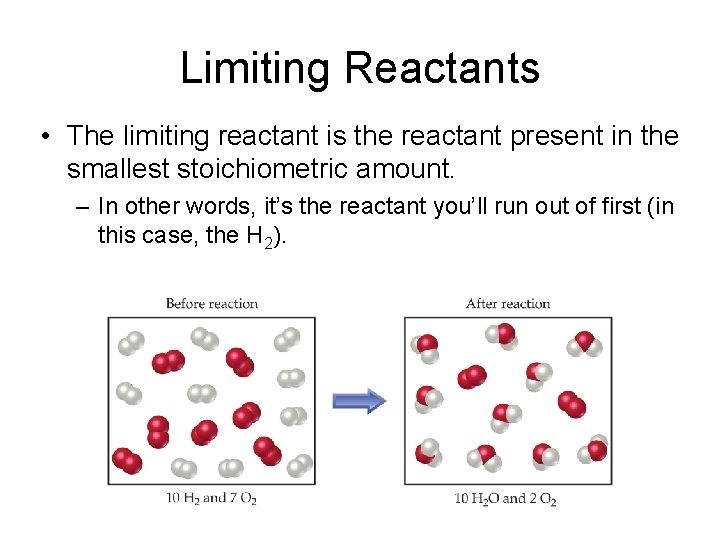
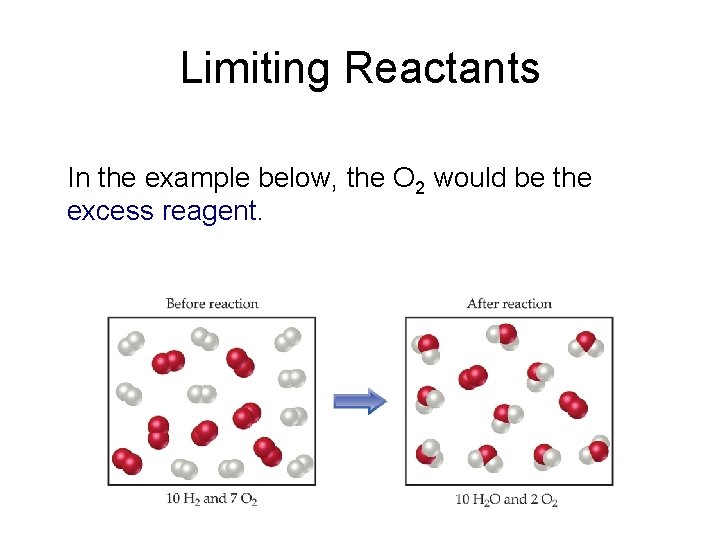
Limiting Reactants • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H 2).

Limiting Reactants In the example below, the O 2 would be the excess reagent.

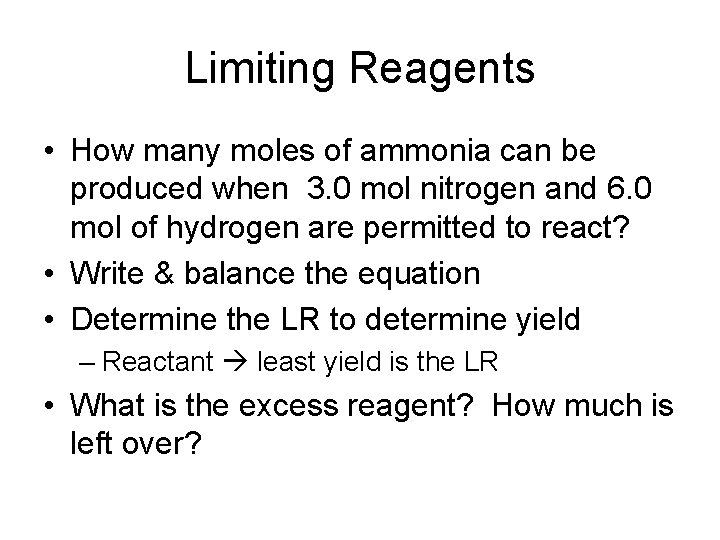
Limiting Reagents • How many moles of ammonia can be produced when 3. 0 mol nitrogen and 6. 0 mol of hydrogen are permitted to react? • Write & balance the equation • Determine the LR to determine yield – Reactant least yield is the LR • What is the excess reagent? How much is left over?

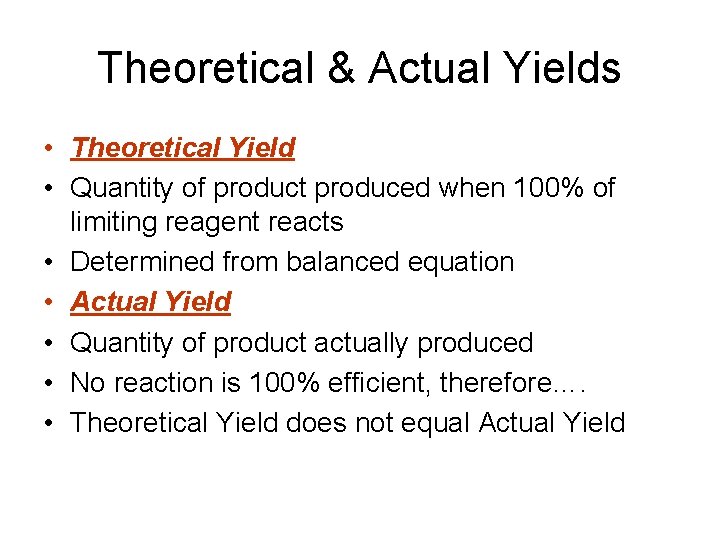
Theoretical & Actual Yields • Theoretical Yield • Quantity of product produced when 100% of limiting reagent reacts • Determined from balanced equation • Actual Yield • Quantity of product actually produced • No reaction is 100% efficient, therefore…. • Theoretical Yield does not equal Actual Yield

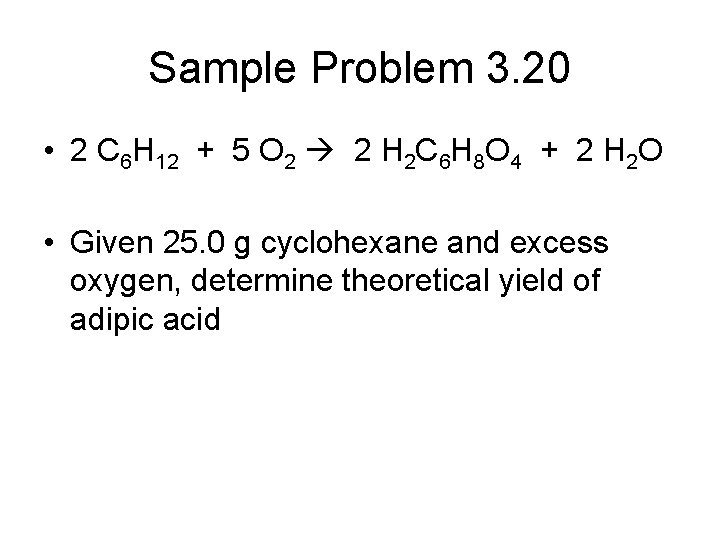
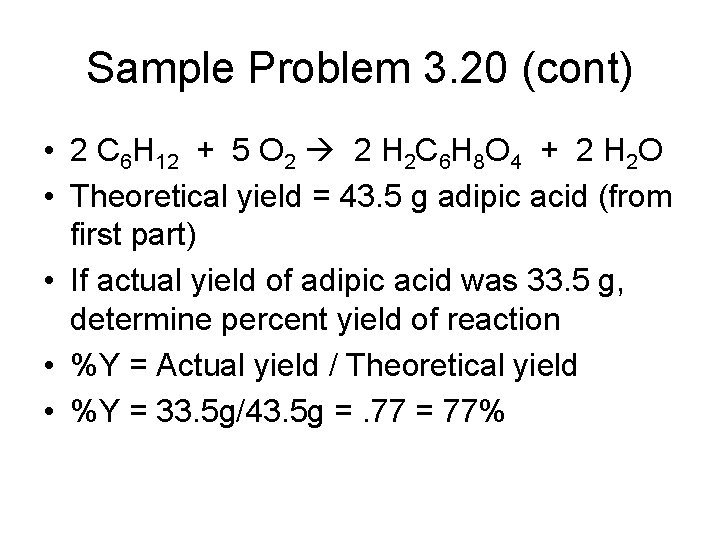
Sample Problem 3. 20 • 2 C 6 H 12 + 5 O 2 2 H 2 C 6 H 8 O 4 + 2 H 2 O • Given 25. 0 g cyclohexane and excess oxygen, determine theoretical yield of adipic acid

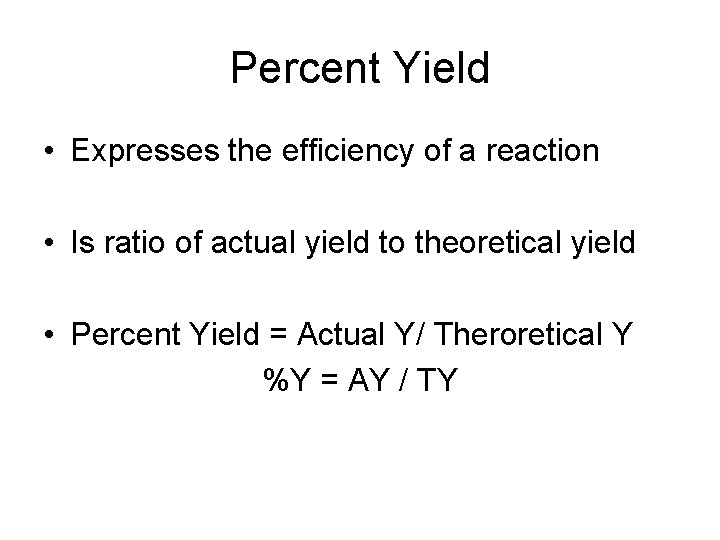
Percent Yield • Expresses the efficiency of a reaction • Is ratio of actual yield to theoretical yield • Percent Yield = Actual Y/ Theroretical Y %Y = AY / TY

Sample Problem 3. 20 (cont) • 2 C 6 H 12 + 5 O 2 2 H 2 C 6 H 8 O 4 + 2 H 2 O • Theoretical yield = 43. 5 g adipic acid (from first part) • If actual yield of adipic acid was 33. 5 g, determine percent yield of reaction • %Y = Actual yield / Theoretical yield • %Y = 33. 5 g/43. 5 g =. 77 = 77%
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of reactions
Types of reactions Mass relationships in chemical reactions
Mass relationships in chemical reactions Stoichiometry island diagram
Stoichiometry island diagram Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Stoichiometry molar mass
Stoichiometry molar mass Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry Stoichiometry predicting amounts in reactions
Stoichiometry predicting amounts in reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet How to write half reactions
How to write half reactions Molar relationships
Molar relationships Stoichiometry refers to
Stoichiometry refers to Chemical accounting: stoichiometry
Chemical accounting: stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Alkyne to alkene
Alkyne to alkene Writing and balancing chemical equations worksheet
Writing and balancing chemical equations worksheet Non aqueous solvents examples
Non aqueous solvents examples What are five chemical changes
What are five chemical changes Percent yield of copper lab
Percent yield of copper lab Describing chemical reactions
Describing chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Describing chemical reactions
Describing chemical reactions 5 types of chemical reactions
5 types of chemical reactions Type of chemical reactions
Type of chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Chemical reactions in soil
Chemical reactions in soil What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Describing chemical reactions
Describing chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions