STOICHIOMETRY LETS REVIEW MOLES AND PARTICLES 1 mole
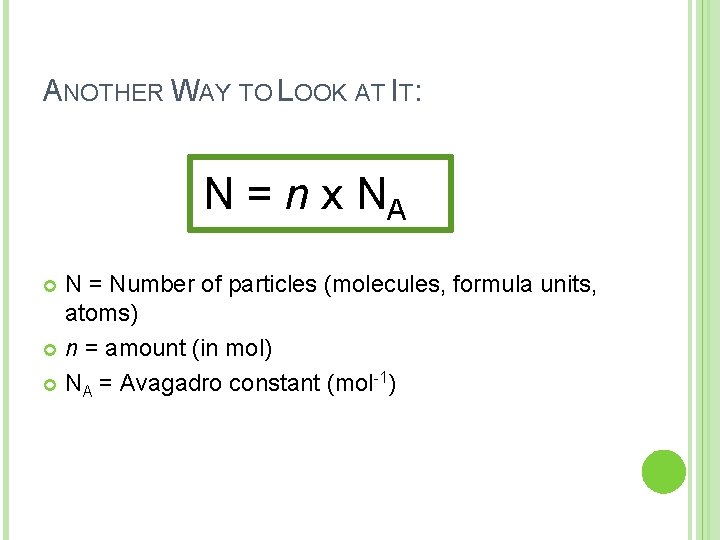







- Slides: 18

STOICHIOMETRY

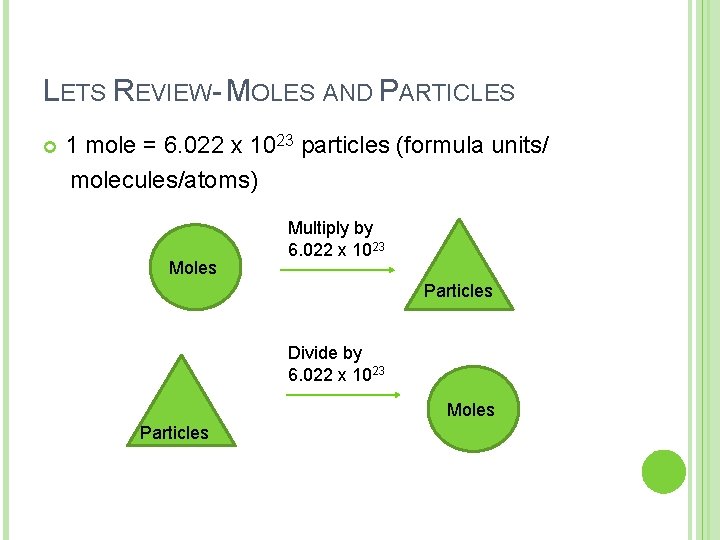
LETS REVIEW- MOLES AND PARTICLES 1 mole = 6. 022 x 1023 particles (formula units/ molecules/atoms) Moles Multiply by 6. 022 x 1023 Particles Divide by 6. 022 x 1023 Moles Particles
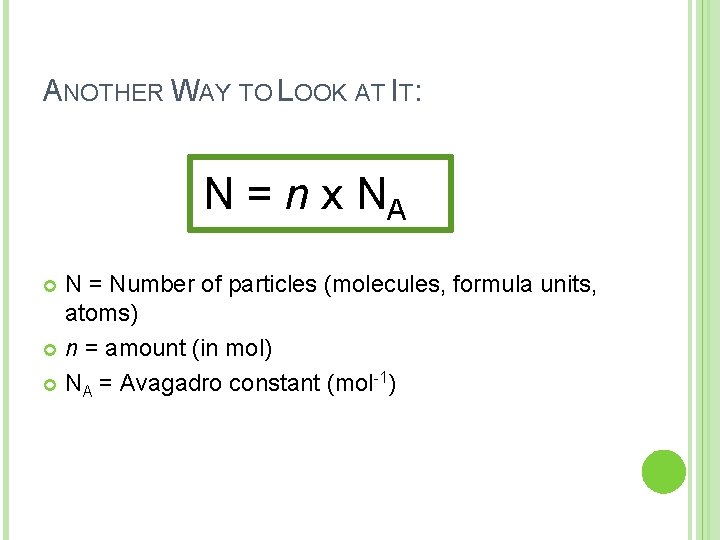
ANOTHER WAY TO LOOK AT IT: N = n x NA N = Number of particles (molecules, formula units, atoms) n = amount (in mol) NA = Avagadro constant (mol-1)

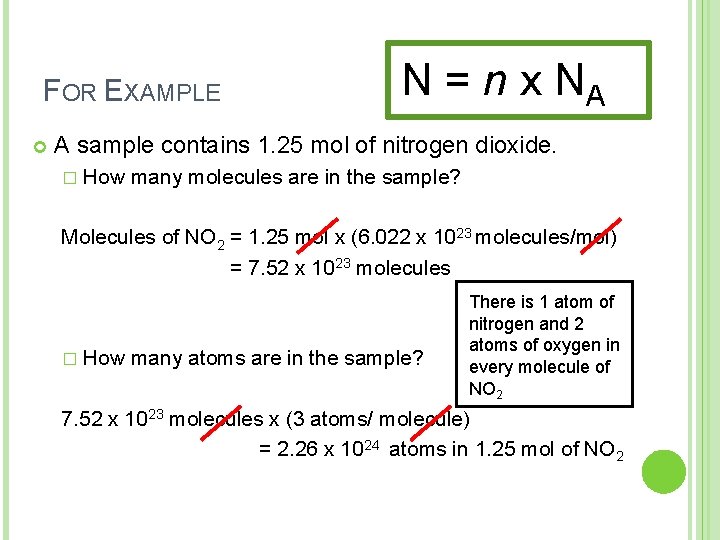
FOR EXAMPLE N = n x NA A sample contains 1. 25 mol of nitrogen dioxide. � How many molecules are in the sample? Molecules of NO 2 = 1. 25 mol x (6. 022 x 1023 molecules/mol) = 7. 52 x 1023 molecules � How many atoms are in the sample? There is 1 atom of nitrogen and 2 atoms of oxygen in every molecule of NO 2 7. 52 x 1023 molecules x (3 atoms/ molecule) = 2. 26 x 10 24 atoms in 1. 25 mol of NO 2

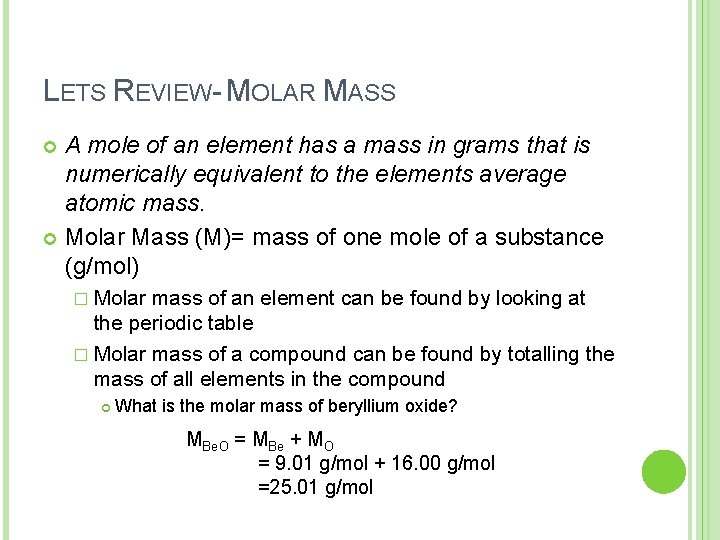
LETS REVIEW- MOLAR MASS A mole of an element has a mass in grams that is numerically equivalent to the elements average atomic mass. Molar Mass (M)= mass of one mole of a substance (g/mol) � Molar mass of an element can be found by looking at the periodic table � Molar mass of a compound can be found by totalling the mass of all elements in the compound What is the molar mass of beryllium oxide? MBe. O = MBe + MO = 9. 01 g/mol + 16. 00 g/mol =25. 01 g/mol

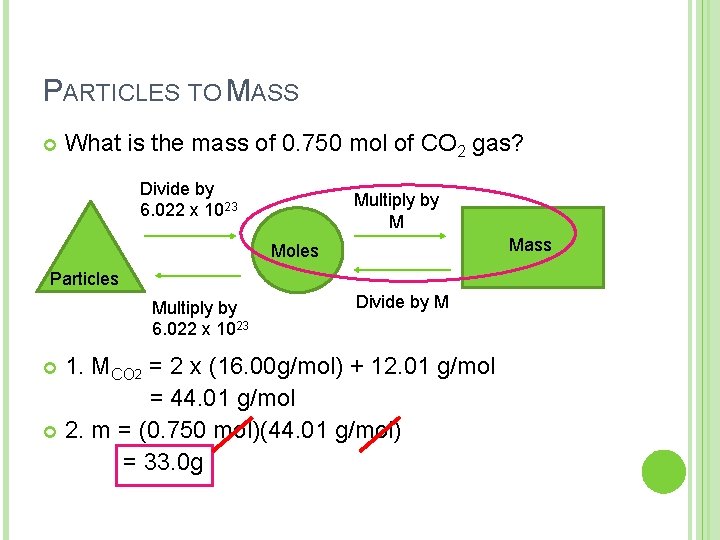
PARTICLES TO MASS What is the mass of 0. 750 mol of CO 2 gas? Divide by 6. 022 x 1023 Multiply by M Mass Moles Particles Multiply by 6. 022 x 1023 Divide by M 1. MCO 2 = 2 x (16. 00 g/mol) + 12. 01 g/mol = 44. 01 g/mol 2. m = (0. 750 mol)(44. 01 g/mol) = 33. 0 g

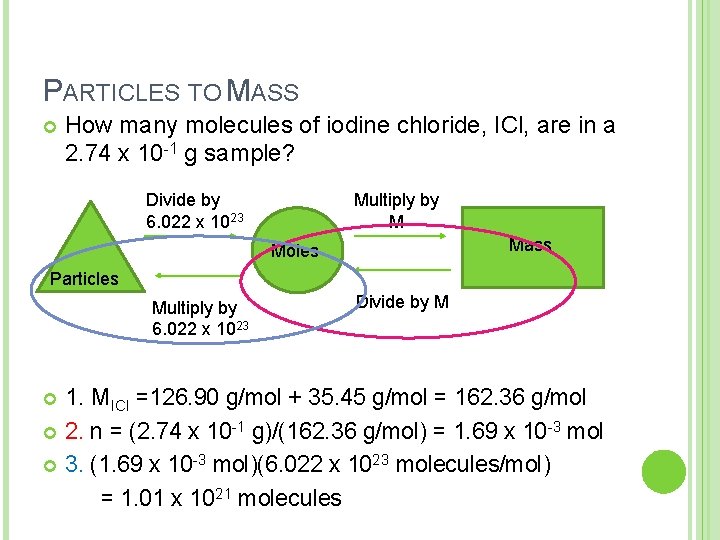
PARTICLES TO MASS How many molecules of iodine chloride, ICl, are in a 2. 74 x 10 -1 g sample? Multiply by M Divide by 6. 022 x 1023 Mass Moles Particles Multiply by 6. 022 x 1023 Divide by M 1. MICl =126. 90 g/mol + 35. 45 g/mol = 162. 36 g/mol 2. n = (2. 74 x 10 -1 g)/(162. 36 g/mol) = 1. 69 x 10 -3 mol 3. (1. 69 x 10 -3 mol)(6. 022 x 1023 molecules/mol) = 1. 01 x 1021 molecules

MOLAR VOLUME

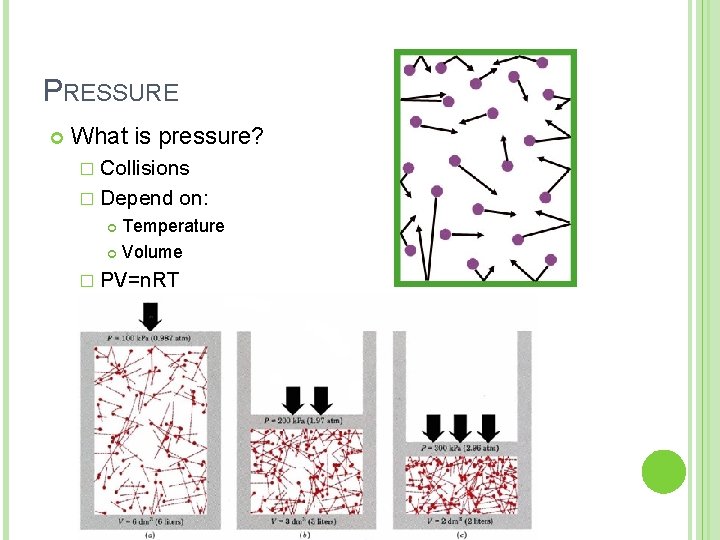
PRESSURE What is pressure? � Collisions � Depend on: Temperature Volume � PV=n. RT

STANDARD TEMPERATURE AND PRESSURE Since V depends on P and T, you must state both when describing a gases V. STP: � Average atmospheric pressure at sea level (101. 3 k. Pa) � Freezing point of water (Oo. C, 273 K)

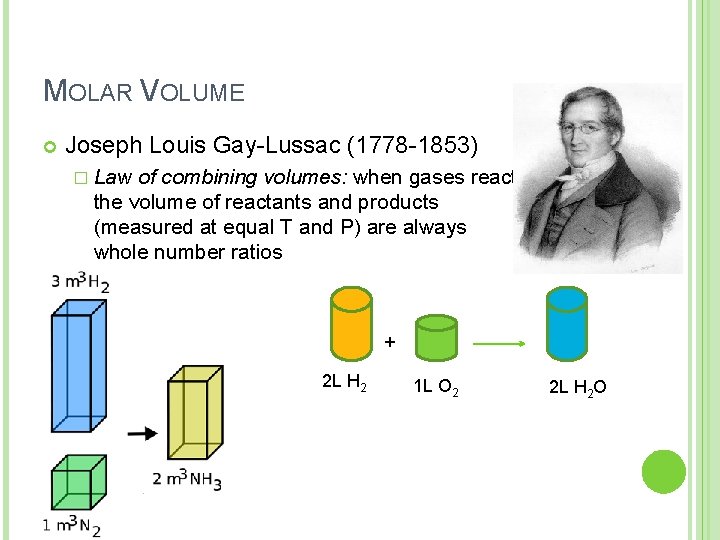
MOLAR VOLUME Joseph Louis Gay-Lussac (1778 -1853) � Law of combining volumes: when gases react the volume of reactants and products (measured at equal T and P) are always whole number ratios + 2 L H 2 1 L O 2 2 L H 2 O

MOLAR VOLUME Amedeo Avogadro (1776 -1856) � Realized he could relate the volume of a gas to the amount that was present (from mass) Avogadro’s Hypothesis: equal volumes of all ideal gases at the same temperature and pressure contain the same number of molecules In other words: 1 mole of any gas has the same volume as 1 mole of any other gas at STP.

MOLAR VOLUME

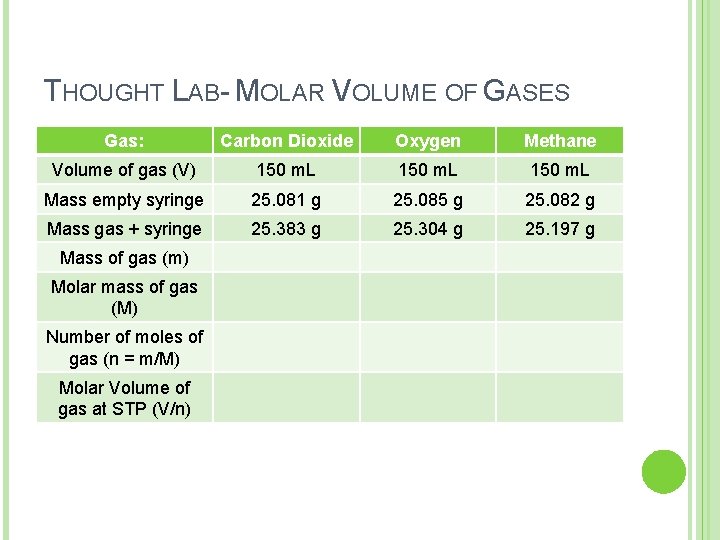
THOUGHT LAB- MOLAR VOLUME OF GASES Two students in a lab decided to calulate the molar volume of carbon dioxide, oxygen and methane gas. They measured the mass of an empty, 150 m. L syringe and then the mass of the syringe + gas. They repeated this procedure for each gas. The experiment was carried out in a room maintained at STP (273 K and 101. 3 k. Pa). Their results are in the table on the next slide:

THOUGHT LAB- MOLAR VOLUME OF GASES Gas: Carbon Dioxide Oxygen Methane Volume of gas (V) 150 m. L Mass empty syringe 25. 081 g 25. 085 g 25. 082 g Mass gas + syringe 25. 383 g 25. 304 g 25. 197 g Mass of gas (m) Molar mass of gas (M) Number of moles of gas (n = m/M) Molar Volume of gas at STP (V/n)

MOLAR VOLUME It turns out that the molar volume of any gas at STP is 22. 4 L/mol! Well, actually, that’s not entirely true…. � The molar volume of 22. 4 L/mol is assumed for ideal gases- which are hypothetical gases that don’t take up space and do not attract one another � Real gases do take up space and attract each other somewhat- so the molar volume would vary slightly from 22. 4 L/mol…. but it’s close � We will always assume the molar volume of a gas is 22. 4 L/mol at STP!

EXAMPLE: What is the volume of 3. 0 mol of nitrogen dioxide gas at STP?

TRY THIS: Suppose you have 44. 8 L of methane gas at STP. � 1. How many moles are present? � 2. What is the mass of the gas? � 3. How many molecules of the gas is present?