Stoichiometry Ideal Stoichiometric Calculations Stoichiometry Objectives Calculate the

Stoichiometry Ideal Stoichiometric Calculations
Stoichiometry Objectives Calculate the amount in moles of a reactant or a product from the amount in moles of a different reactant or product Calculate the mass of a reactant or a product from the amount in moles of a different reactant or product Calculate the amount in moles of a reactant or a product from the mass of a different reactant or product Calculate the mass of a reactant or a product from the mass of a different reactant or product
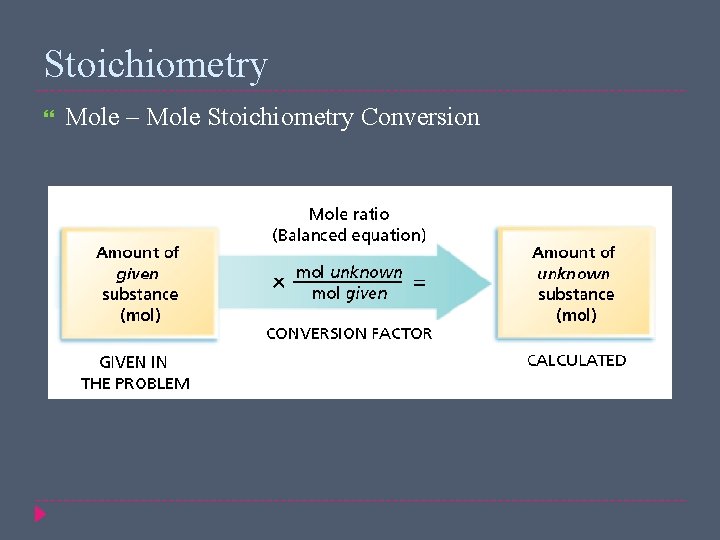
Stoichiometry Mole – Mole Stoichiometry Conversion
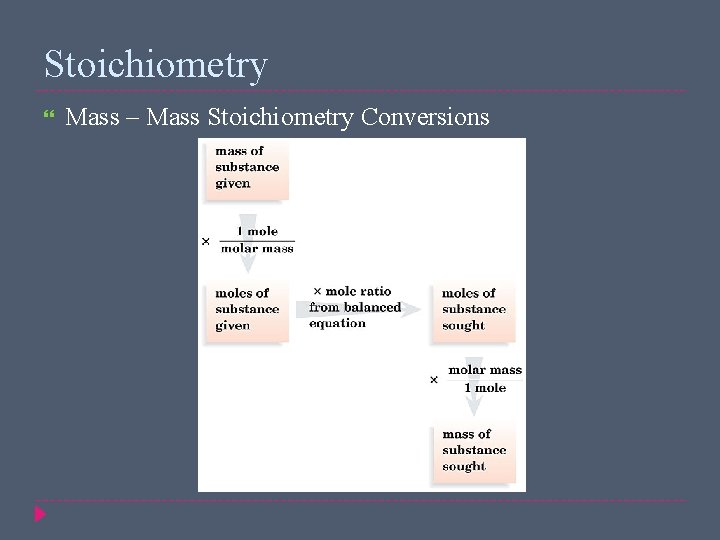
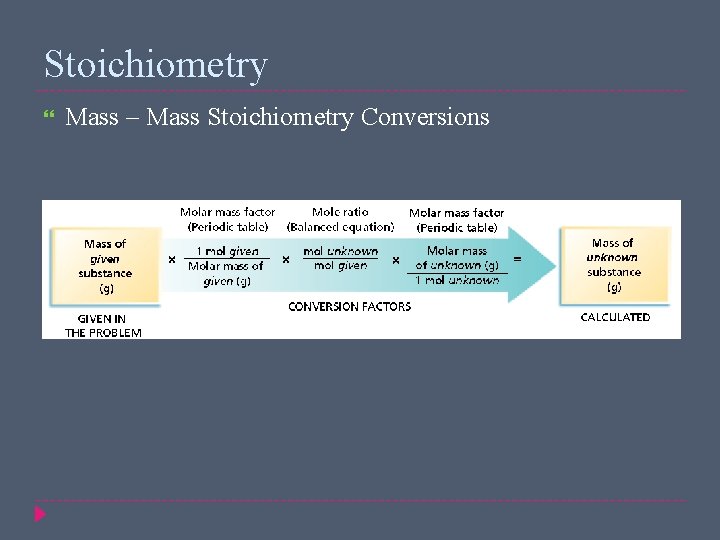
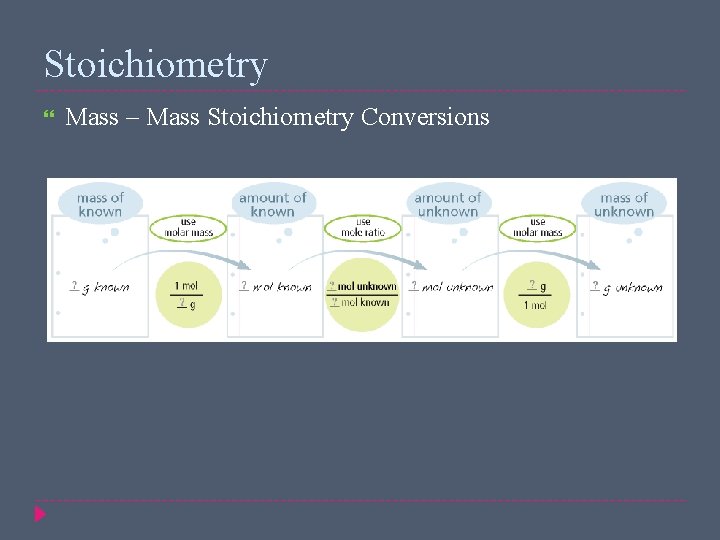
Stoichiometry Mass – Mass Stoichiometry Conversions
Stoichiometry Mole – Mole Stoichiometry Conversions Sample Problem In a spacecraft, the carbon dioxide exhaled by astronauts can be removed by its reaction with lithium hydroxide, Li. OH, according to the following chemical equation CO 2(g) + 2 Li. OH(s) → Li 2 CO 3(s) + H 2 O(l) How many moles of lithium hydroxide are required to react with 20 mol CO 2, the average amount exhaled by a person each day?
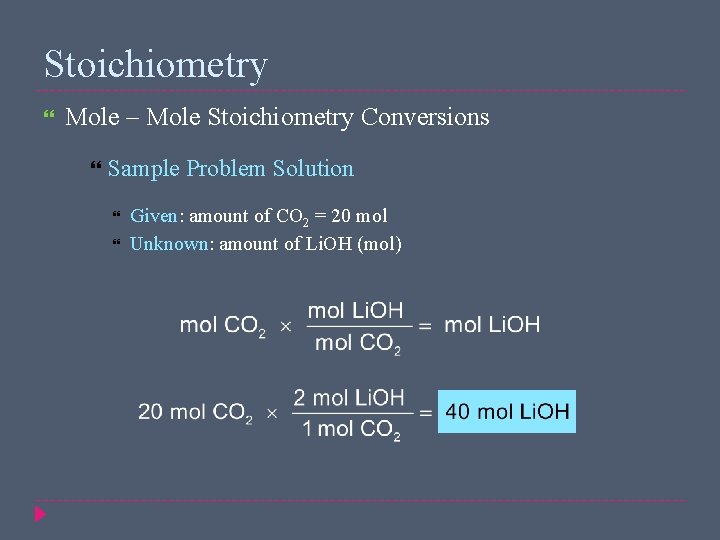
Stoichiometry Mole – Mole Stoichiometry Conversions Sample Problem Solution Given: amount of CO 2 = 20 mol Unknown: amount of Li. OH (mol)
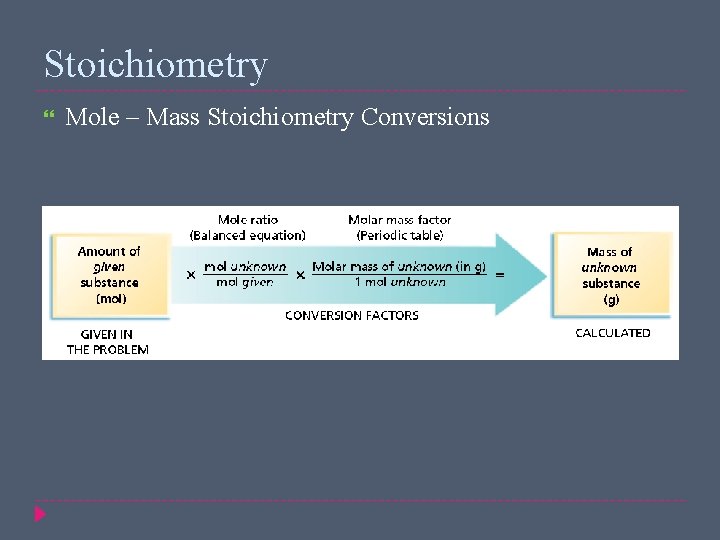
Stoichiometry Mole – Mass Stoichiometry Conversions
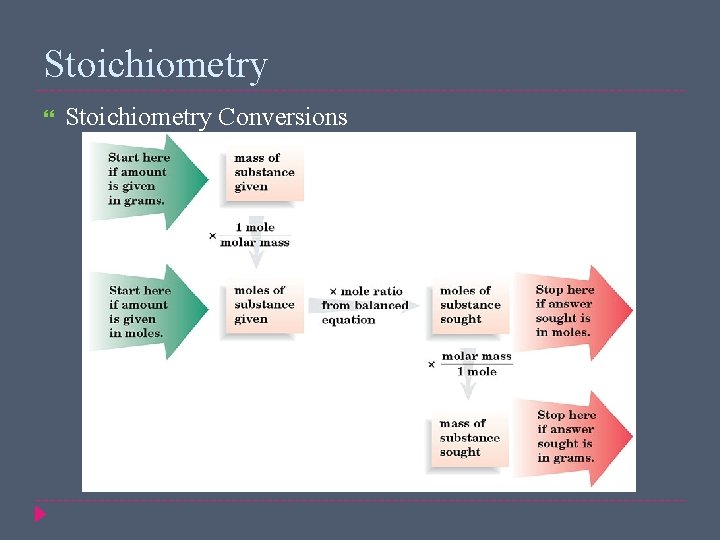
Stoichiometry Conversions
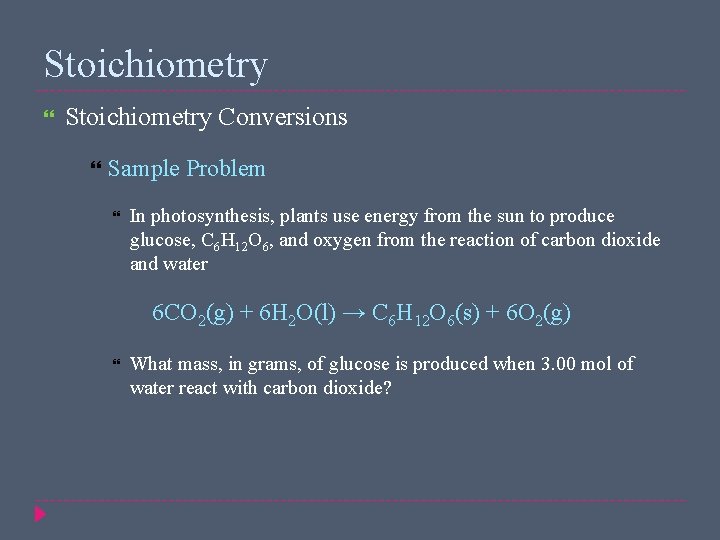
Stoichiometry Conversions Sample Problem In photosynthesis, plants use energy from the sun to produce glucose, C 6 H 12 O 6, and oxygen from the reaction of carbon dioxide and water 6 CO 2(g) + 6 H 2 O(l) → C 6 H 12 O 6(s) + 6 O 2(g) What mass, in grams, of glucose is produced when 3. 00 mol of water react with carbon dioxide?
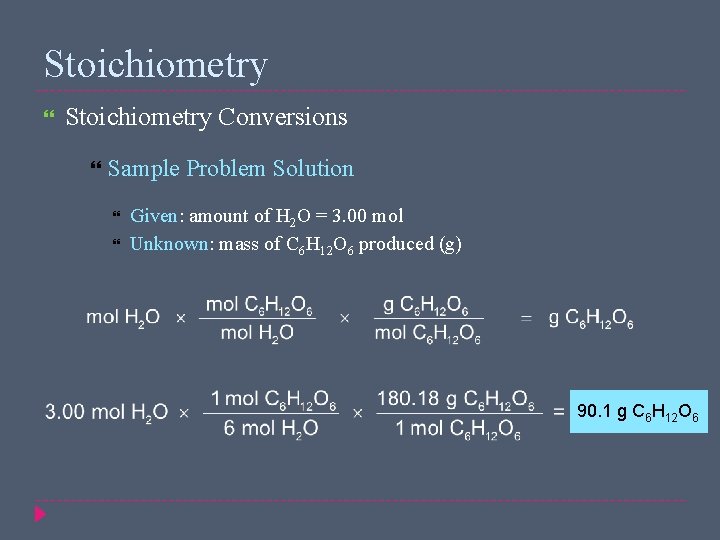
Stoichiometry Conversions Sample Problem Solution Given: amount of H 2 O = 3. 00 mol Unknown: mass of C 6 H 12 O 6 produced (g) 90. 1 g C 6 H 12 O 6
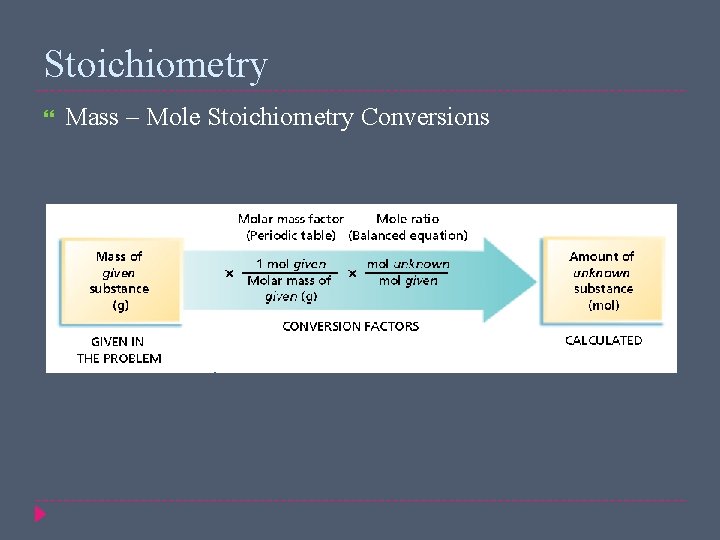
Stoichiometry Mass – Mole Stoichiometry Conversions
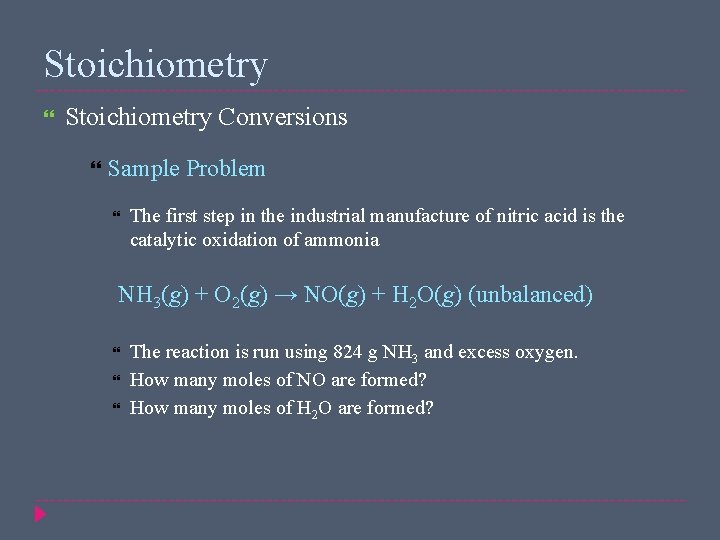
Stoichiometry Conversions Sample Problem The first step in the industrial manufacture of nitric acid is the catalytic oxidation of ammonia NH 3(g) + O 2(g) → NO(g) + H 2 O(g) (unbalanced) The reaction is run using 824 g NH 3 and excess oxygen. How many moles of NO are formed? How many moles of H 2 O are formed?
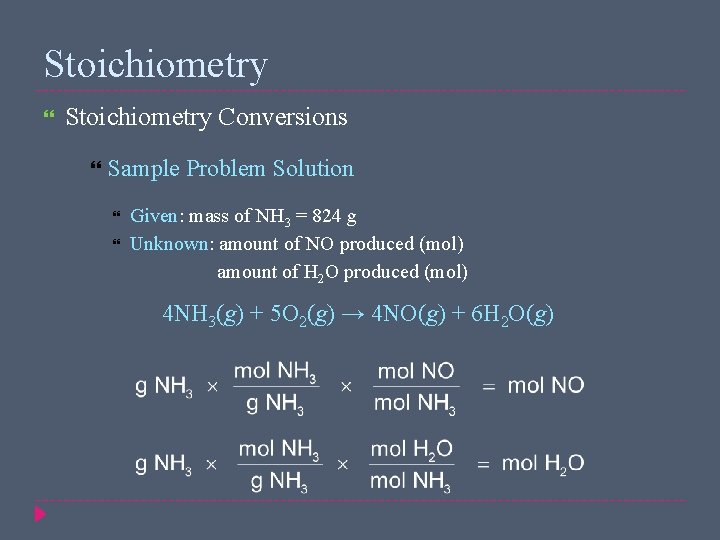
Stoichiometry Conversions Sample Problem Solution Given: mass of NH 3 = 824 g Unknown: amount of NO produced (mol) amount of H 2 O produced (mol) 4 NH 3(g) + 5 O 2(g) → 4 NO(g) + 6 H 2 O(g)
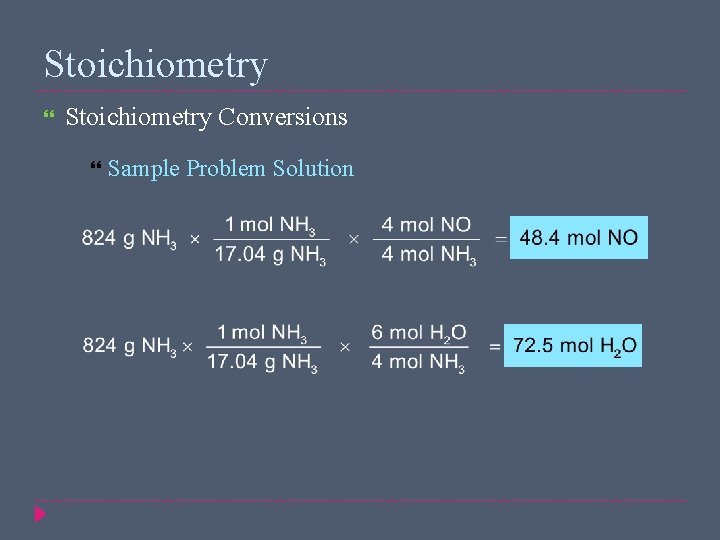
Stoichiometry Conversions Sample Problem Solution
Stoichiometry Mass – Mass Stoichiometry Conversions
Stoichiometry Mass – Mass Stoichiometry Conversions
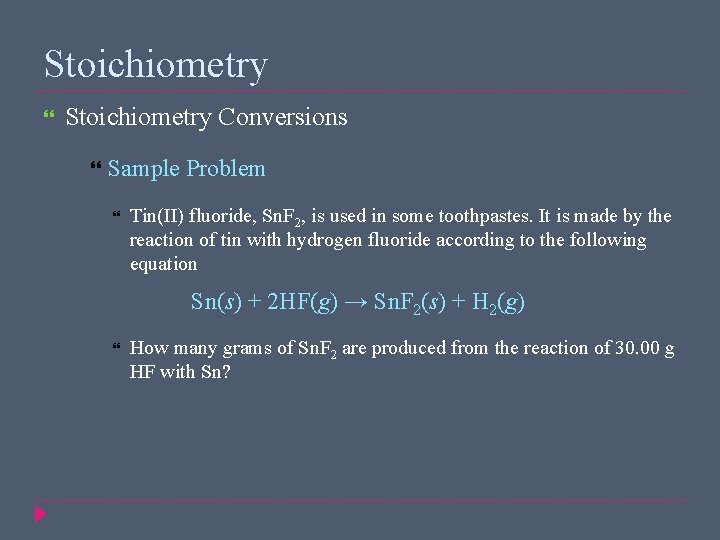
Stoichiometry Conversions Sample Problem Tin(II) fluoride, Sn. F 2, is used in some toothpastes. It is made by the reaction of tin with hydrogen fluoride according to the following equation Sn(s) + 2 HF(g) → Sn. F 2(s) + H 2(g) How many grams of Sn. F 2 are produced from the reaction of 30. 00 g HF with Sn?
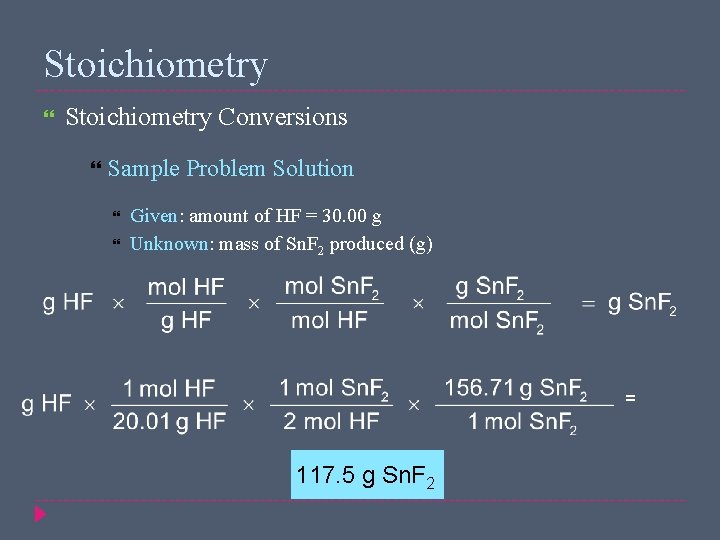
Stoichiometry Conversions Sample Problem Solution Given: amount of HF = 30. 00 g Unknown: mass of Sn. F 2 produced (g) = 117. 5 g Sn. F 2
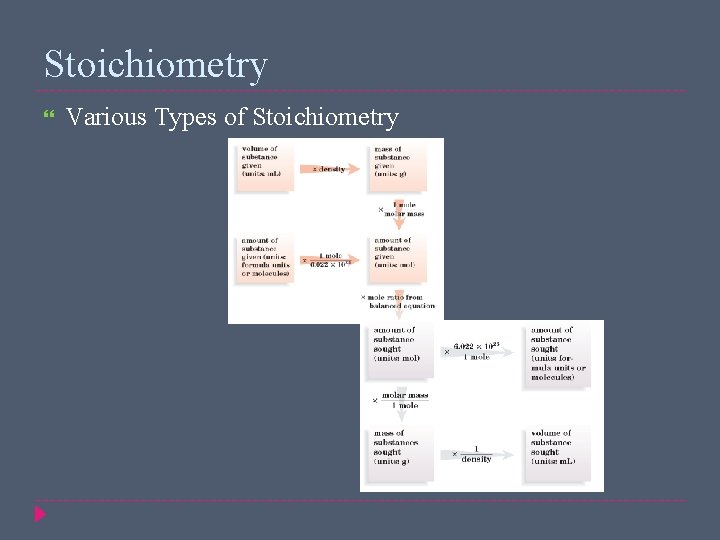
Stoichiometry Various Types of Stoichiometry
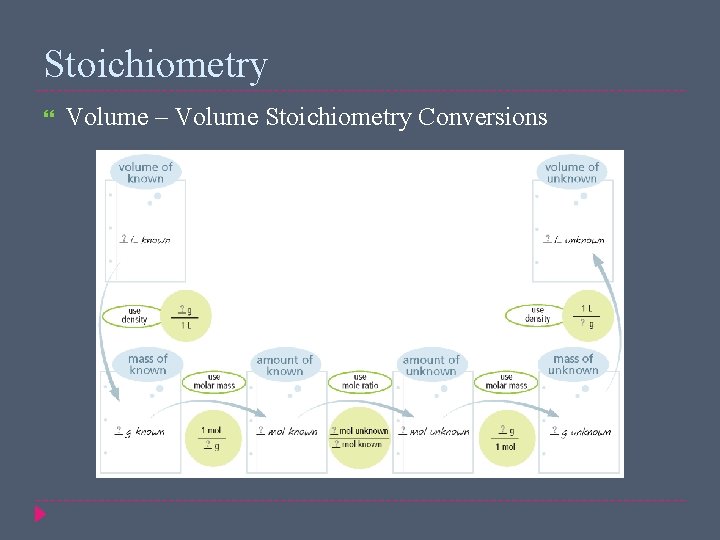
Stoichiometry Volume – Volume Stoichiometry Conversions
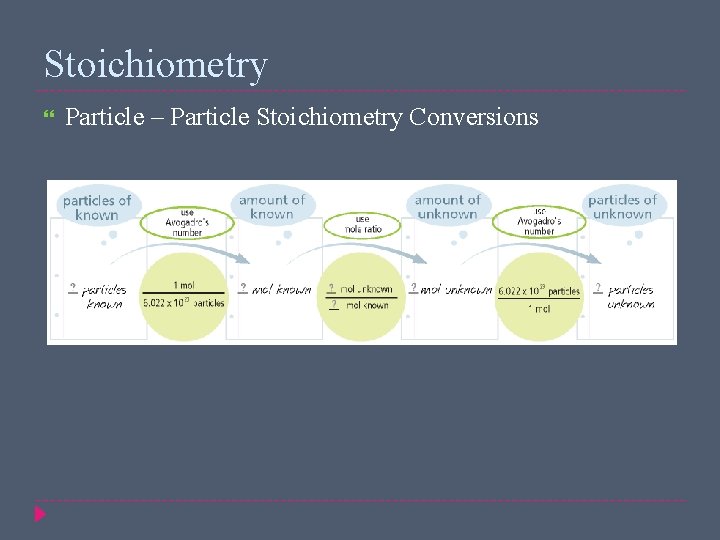
Stoichiometry Particle – Particle Stoichiometry Conversions
- Slides: 21