STOICHIOMETRY DAY 1 Converting from one substance to
STOICHIOMETRY – DAY 1 (Converting from one substance to a DIFFERENT substance) Moles → Moles & Grams → Grams 1

What is Stoichiometry? ? ? Convert from one substance to a DIFFERENT substance!!! Definition: Definition to predict the amount of reactant and/or product that will be used or produced in a chemical reaction. When doing stoichiometry, the chemical equation MUST BE BALANCED!!! 2
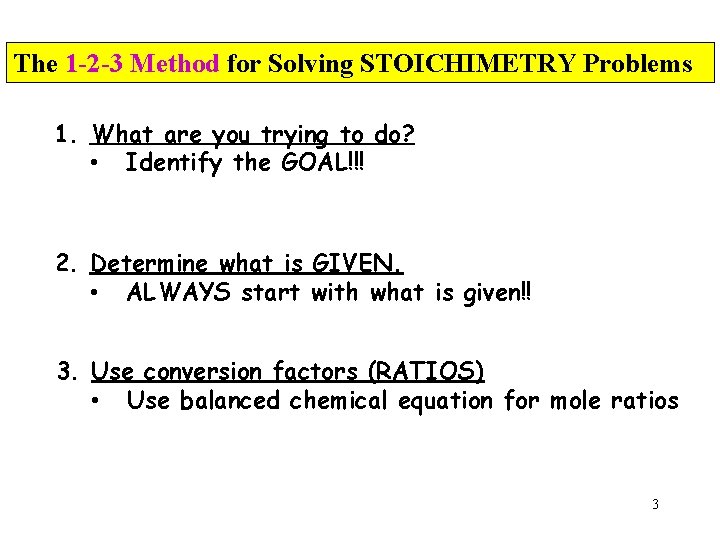
The 1 -2 -3 Method for Solving STOICHIMETRY Problems 1. What are you trying to do? • Identify the GOAL!!! 2. Determine what is GIVEN. • ALWAYS start with what is given!! 3. Use conversion factors (RATIOS) • Use balanced chemical equation for mole ratios 3
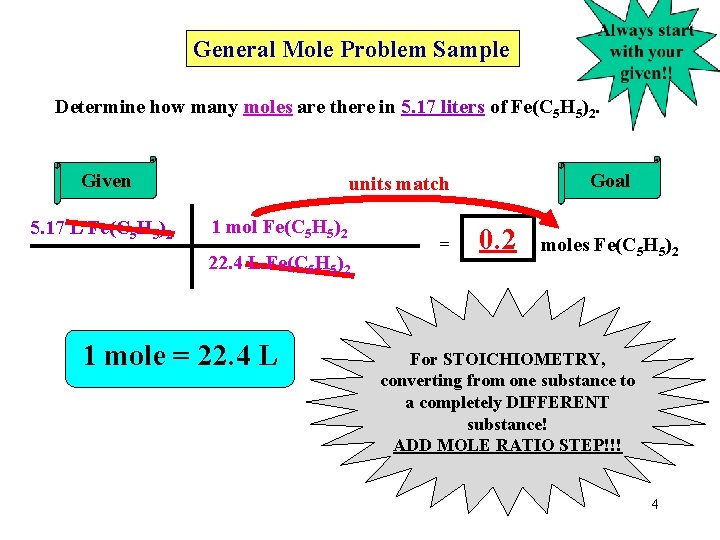
General Mole Problem Sample Determine how many moles are there in 5. 17 liters of Fe(C 5 H 5)2. Given 5. 17 L Fe(C 5 H 5)2 Goal units match 1 mol Fe(C 5 H 5)2 22. 4 L Fe(C 5 H 5)2 1 mole = 22. 4 L = 0. 2 moles Fe(C 5 H 5)2 For STOICHIOMETRY, converting from one substance to a completely DIFFERENT substance! ADD MOLE RATIO STEP!!! 4
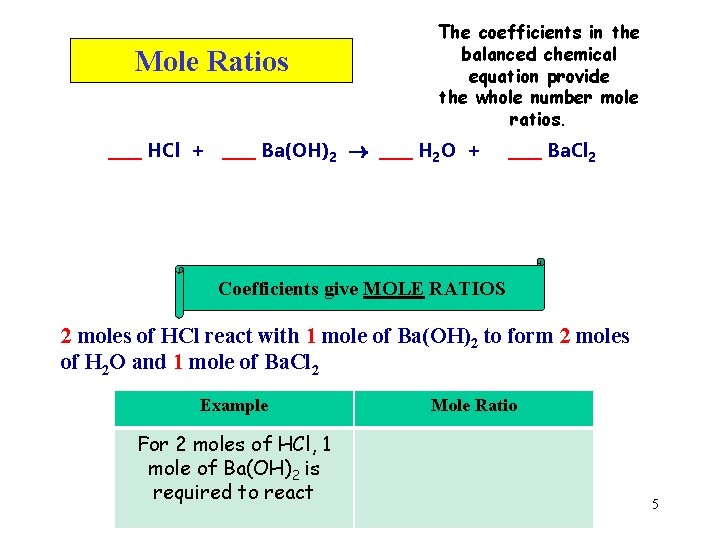
Mole Ratios The coefficients in the balanced chemical equation provide the whole number mole ratios. ____ HCl + ____ Ba(OH)2 ____ H 2 O + ____ Ba. Cl 2 Coefficients give MOLE RATIOS 2 moles of HCl react with 1 mole of Ba(OH)2 to form 2 moles of H 2 O and 1 mole of Ba. Cl 2 Example For 2 moles of HCl, 1 mole of Ba(OH)2 is required to react Mole Ratio 5
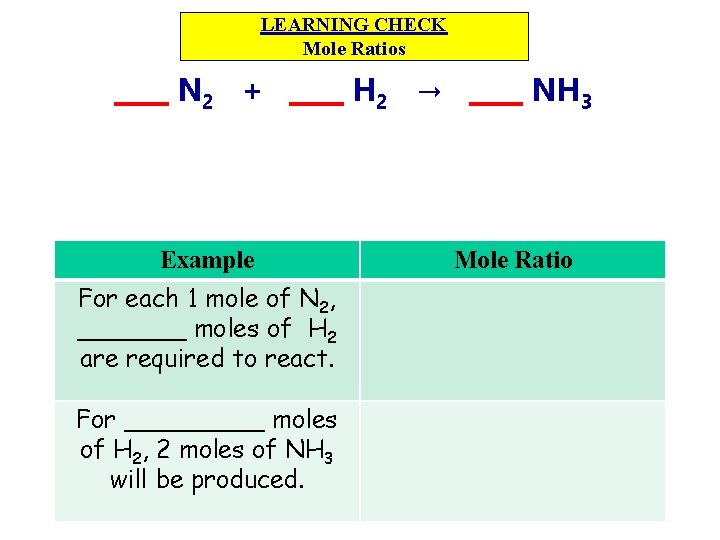
LEARNING CHECK Mole Ratios ____ N 2 + ____ H 2 → ____ NH 3 Example Mole Ratio For each 1 mole of N 2, _______ moles of H 2 are required to react. For _____ moles of H 2, 2 moles of NH 3 will be produced. 6
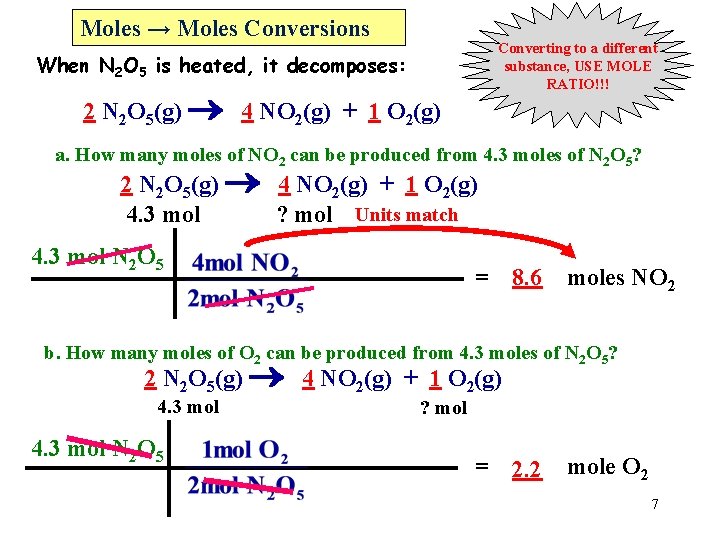
Moles → Moles Conversions Converting to a different substance, USE MOLE RATIO!!! When N 2 O 5 is heated, it decomposes: 2 N 2 O 5(g) 4 NO 2(g) + 1 O 2(g) a. How many moles of NO 2 can be produced from 4. 3 moles of N 2 O 5? 2 N 2 O 5(g) 4 NO 2(g) + 1 O 2(g) 4. 3 mol ? mol Units match 4. 3 mol N 2 O 5 = 8. 6 moles NO 2 b. How many moles of O 2 can be produced from 4. 3 moles of N 2 O 5? 2 N 2 O 5(g) 4 NO 2(g) + 1 O 2(g) 4. 3 mol N 2 O 5 ? mol = 2. 2 mole O 2 7

LEARNING CHECK Mole → Mole 2 Pb. O 2 → Converting to a different substance, USE MOLE RATIO!!! 2 Pb. O + 1 O 2 Using the balanced equation above, how many moles of Pb. O 2 where used if 2. 3 moles of Pb. O was produced. 8
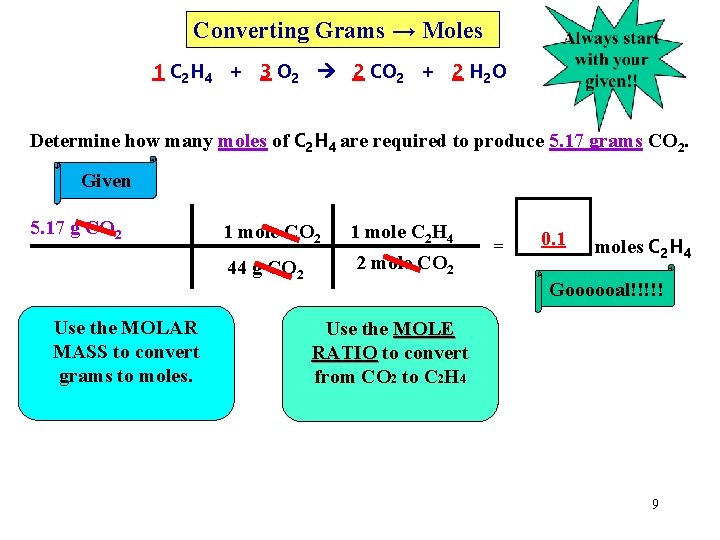
Converting Grams → Moles 1 C 2 H 4 + 3 O 2 2 CO 2 + 2 H 2 O Determine how many moles of C 2 H 4 are required to produce 5. 17 grams CO 2. Given 5. 17 g CO 2 1 mole CO 2 44 g CO 2 Use the MOLAR MASS to convert grams to moles. 1 mole C 2 H 4 2 mole CO 2 = 0. 1 moles C 2 H 4 Goooooal!!!!! Use the MOLE RATIO to convert from CO 2 to C 2 H 4 9

LEARNING CHECK Grams → Moles Converting to a different substance, USE MOLE RATIO!!! 10
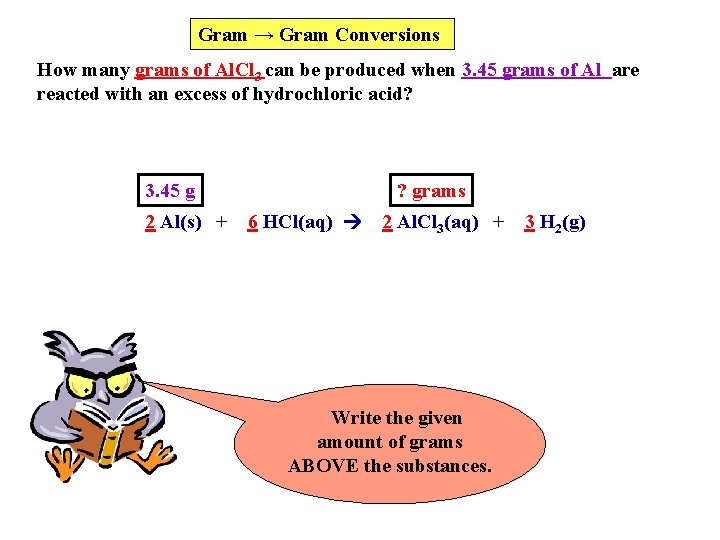
Gram → Gram Conversions How many grams of Al. Cl 3 can be produced when 3. 45 grams of Al are reacted with an excess of hydrochloric acid? 3. 45 g 2 Al(s) + ? grams 6 HCl(aq) 2 Al. Cl 3(aq) + Write the given amount of grams ABOVE the substances. 3 H 2(g)
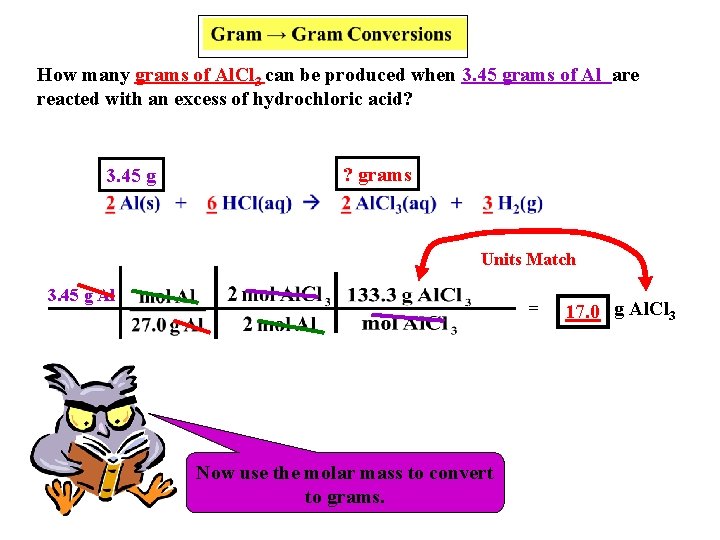
How many grams of Al. Cl 3 can be produced when 3. 45 grams of Al are reacted with an excess of hydrochloric acid? 3. 45 g ? grams Units Match 3. 45 g Al = Now We must Now use Let’s the always use work molar thethe convert molar mass problem. ratio. to toconvert moles. to grams. 17. 0 g Al. Cl 3
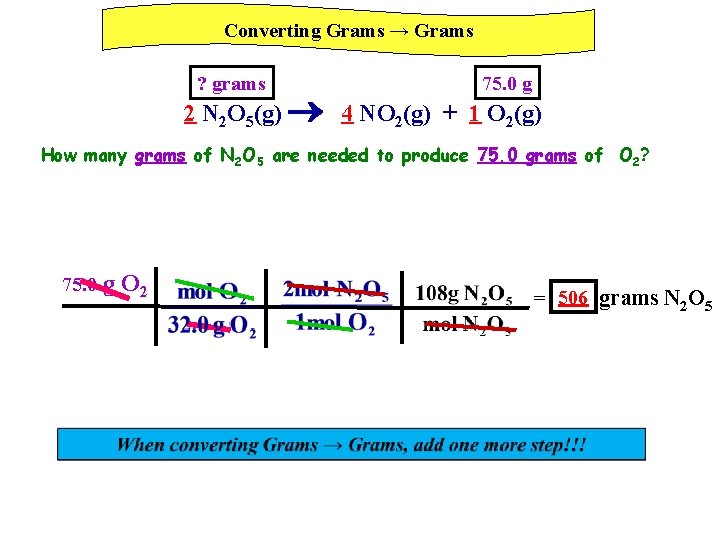
Converting Grams → Grams ? grams 75. 0 g 2 N 2 O 5(g) 4 NO 2(g) + 1 O 2(g) How many grams of N 2 O 5 are needed to produce 75. 0 grams of O 2? 75. 0 g O 2 = 506 grams N 2 O 5
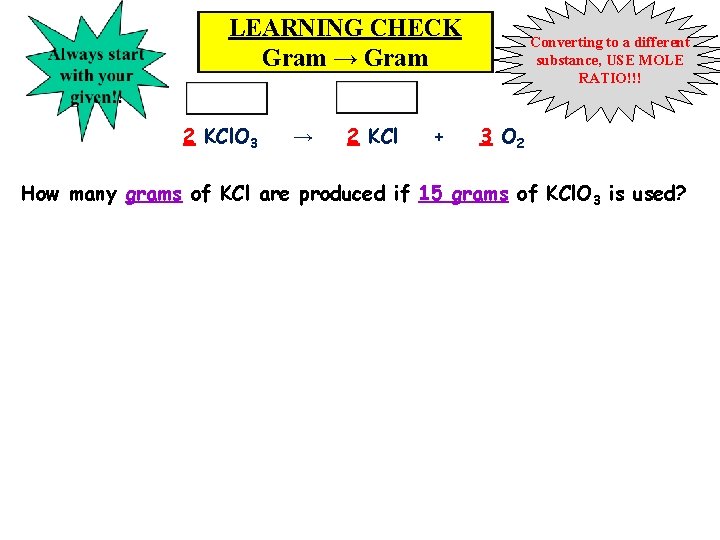
LEARNING CHECK Gram → Gram 2 KCl. O 3 → 2 KCl + Converting to a different substance, USE MOLE RATIO!!! 3 O 2 How many grams of KCl are produced if 15 grams of KCl. O 3 is used?
- Slides: 14