Stoichiometry CHAPTER 10 Stoichiometry is the mass and













- Slides: 19

Stoichiometry CHAPTER 10

Stoichiometry is the mass and amount relationship of reactant and products. Consider the following reaction ; 4 NH 3(g) + 5 O 2(g)--- 4 NO(g) +6 H 2 O(g) The equation states that 4 mol ammonia react with 5 mol oxygen to produce 4 mol NO and 6 mol H 2 O. Suppose you have 2. 4 mol of NH 3. How many mol O 2 are needed?

` First you have to carry a mole to mole conversion. mol NH 3 - mol O 2 2. 4 mol of ammonia х 5 mol of oxygen/4 mol of ammonia =3. 0 mol of oxygen.

Class Practice You are given 450 g of iso amyl alcohol(C 5 H 11 OH). Convert to moles of iso amyl alcohol?

Mass to mass problem solving plan 1. Always begin with a balanced chemical equation. 2. Use the molar mass of the given substance to convert from mass of given to product of given. 3. Use the mole ratio of coefficients from the balanced chemical reaction to change from amount of given to amount of unknown. 4. Use the molar mass of unknown to convert from amount of unknown to mass of unknown.

Mole to mass problem solving plan Always begin with a balanced chemical equation. Use the mole ratio of coefficients from the balanced chemical reaction to change from amount of given to amount of unknown. Use the molar mass of unknown to convert from amount of unknown to mass of unknown.

Homework Page 356 4 and 5

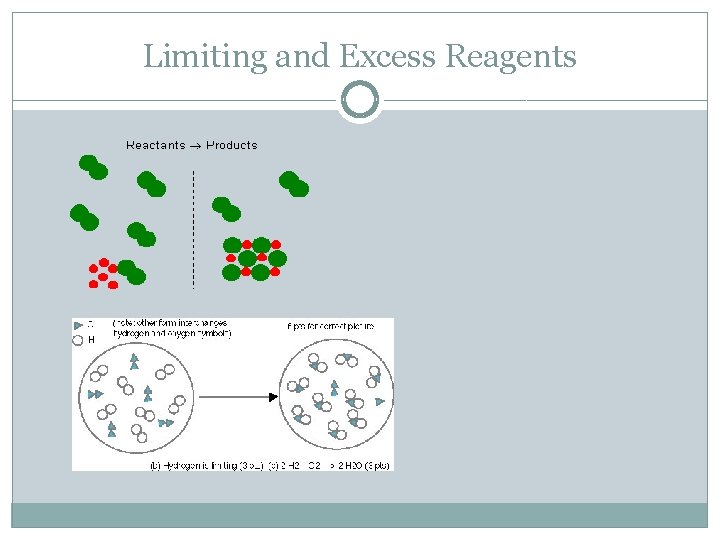
Limiting reactant, excess reactant Limiting Reactant - The reactant in a chemical reaction that limits the amount of product that can be formed. The reaction will stop when all of the limiting reactant is consumed. Excess Reactant - The reactant in a chemical reaction that remains when a reaction stops when the limiting reactant is completely consumed. The excess reactant remains because there is nothing with which it can react.

Limiting and Excess Reagents

Class Practice Carbon monoxide can be combined with hydrogen to produce methanol, CH 3 OH. If you had 152. 5 g CO and 24. 50 g H 2. What mass of CH 3 OH could be produced?

Home work Page 360 1 and 2

Yields The mass of product expected from stochiometric calculations is called theoretical yield. Actual yield is the mass of the product actually obtained. This is usually less than the actual yield. The ratio of actual yield to theoretical yield multiplied by 100 is the percentage yield.

Class Practice A student is synthesizing aspirin by adding 200. 0 g of salicylic acid to an excess of acetic anhydride. Calculate the percentage yield if 231 g of aspirin is produced.

Home work Page 366 4, 6, 7

Application of stochiometry Stochiometry has a number of applications, from banana flavoring, to cosmetics to aspirin. One important application of stochiometry is in air bags used in cars. The function of the airbags is to protect the occupant from injuring themselves by hitting against the windshield or steering wheel or the instrument panel. Stochiometry is used to make sure the airbags do not overinflate or underinflate. Under inflation would not protect the occupant and overinflation may cause the bag to rupture making them useless.

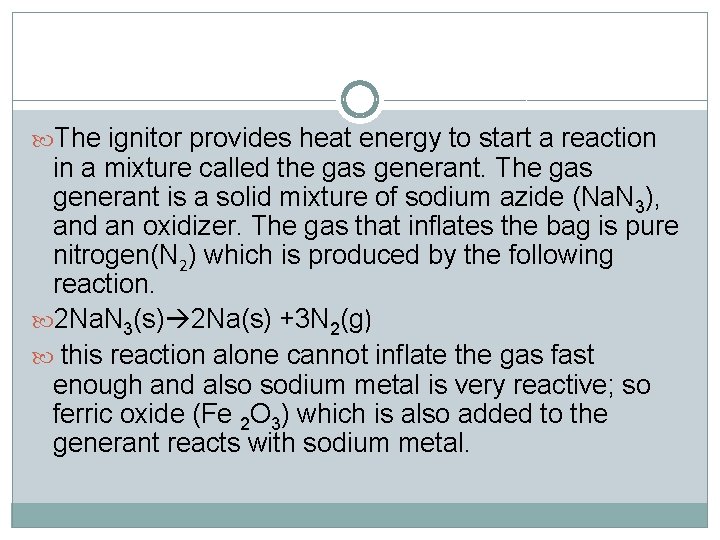
The ignitor provides heat energy to start a reaction in a mixture called the gas generant. The gas generant is a solid mixture of sodium azide (Na. N 3), and an oxidizer. The gas that inflates the bag is pure nitrogen(N 2) which is produced by the following reaction. 2 Na. N 3(s) 2 Na(s) +3 N 2(g) this reaction alone cannot inflate the gas fast enough and also sodium metal is very reactive; so ferric oxide (Fe 2 O 3) which is also added to the generant reacts with sodium metal.

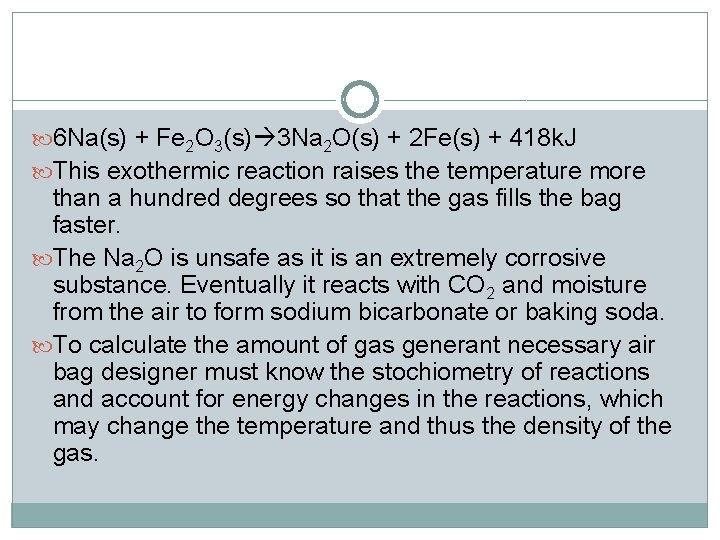
6 Na(s) + Fe 2 O 3(s) 3 Na 2 O(s) + 2 Fe(s) + 418 k. J This exothermic reaction raises the temperature more than a hundred degrees so that the gas fills the bag faster. The Na 2 O is unsafe as it is an extremely corrosive substance. Eventually it reacts with CO 2 and moisture from the air to form sodium bicarbonate or baking soda. To calculate the amount of gas generant necessary air bag designer must know the stochiometry of reactions and account for energy changes in the reactions, which may change the temperature and thus the density of the gas.

Class Practice Assume that 65. 1 L of N₂ gas is needed to inflate an air bag to the proper size. What mass of Na. N₃ must be included in the gas generant to generate this volume of N₂?

Home work Page 378 15, 16, 17