Stoichiometry Ch 11 II Stoichiometry in the Real



- Slides: 12

Stoichiometry – Ch. 11 II. Stoichiometry in the Real World (p. 367 -397)

A. Limiting Reactants b. Available Ingredients • 4 slices of bread • 1 jar of peanut butter • 1/2 jar of jelly b. Limiting Reactant • bread b. Excess Reactants • peanut butter and jelly

A. Limiting Reactants b. Limiting Reactant • used up in a reaction • determines the amount of product b. Excess Reactant • added to ensure that the other reactant is completely used up • cheaper & easier to recycle

A. Limiting Reactants 1. Write a balanced equation. 2. For each reactant, calculate the amount of product formed. 3. Smaller answer indicates: • limiting reactant • amount of product

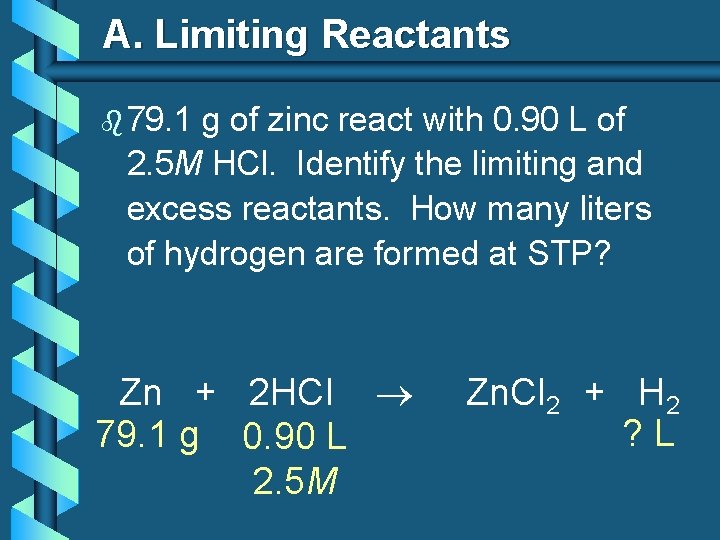
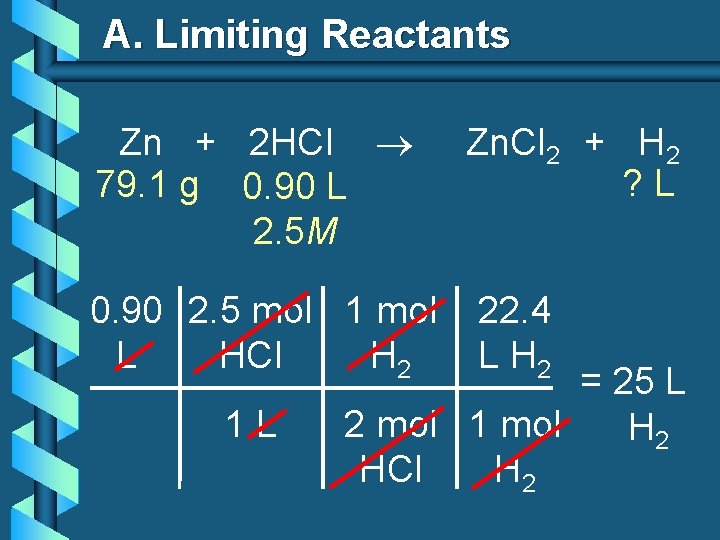
A. Limiting Reactants b 79. 1 g of zinc react with 0. 90 L of 2. 5 M HCl. Identify the limiting and excess reactants. How many liters of hydrogen are formed at STP? Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M Zn. Cl 2 + H 2 ? L

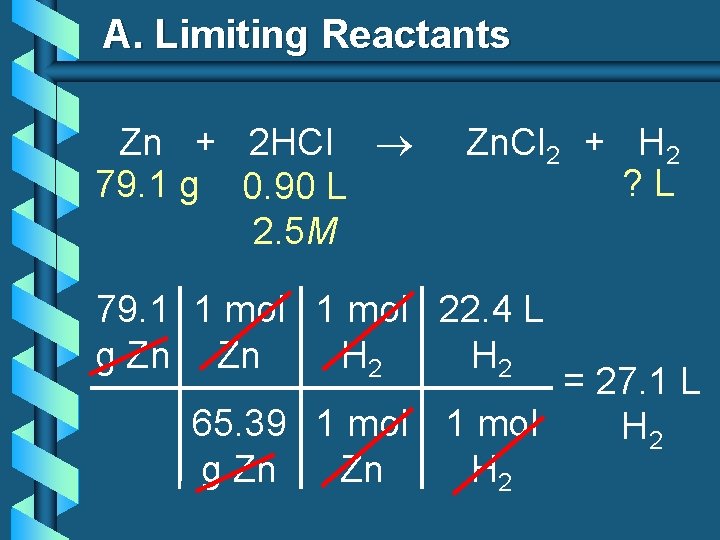
A. Limiting Reactants Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M Zn. Cl 2 + H 2 ? L 79. 1 1 mol 22. 4 L g Zn Zn H 2 = 27. 1 L 65. 39 1 mol H 2 g Zn Zn H 2

A. Limiting Reactants Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M 0. 90 2. 5 mol 1 mol L HCl H 2 1 L Zn. Cl 2 + H 2 ? L 22. 4 L H 2 = 25 L 2 mol 1 mol H 2 HCl H 2

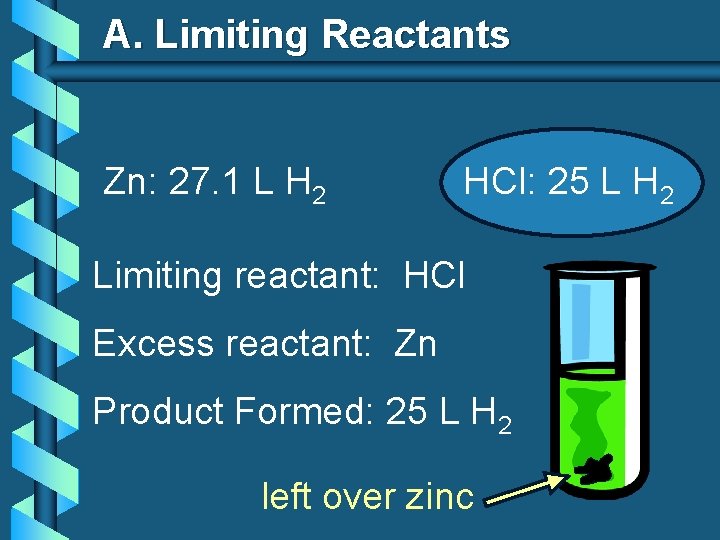
A. Limiting Reactants Zn: 27. 1 L H 2 HCl: 25 L H 2 Limiting reactant: HCl Excess reactant: Zn Product Formed: 25 L H 2 left over zinc

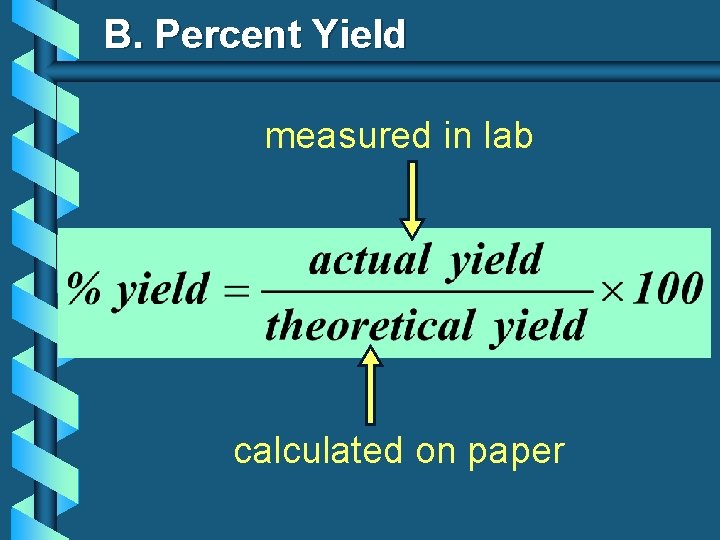
B. Percent Yield measured in lab calculated on paper

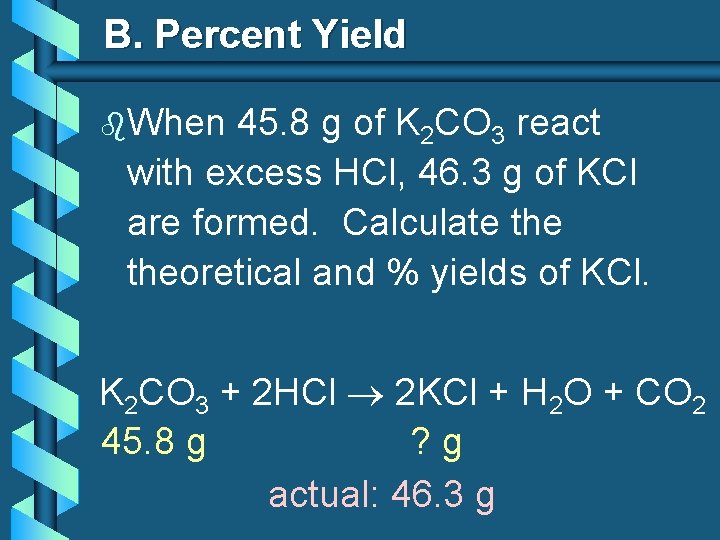
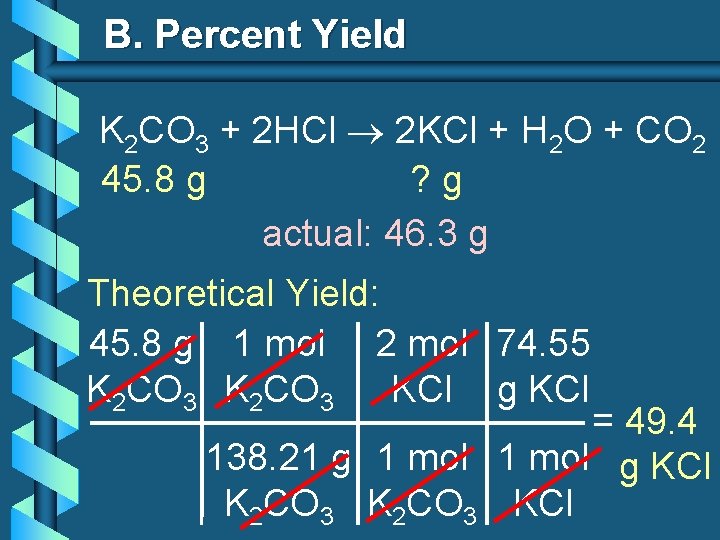
B. Percent Yield b. When 45. 8 g of K 2 CO 3 react with excess HCl, 46. 3 g of KCl are formed. Calculate theoretical and % yields of KCl. K 2 CO 3 + 2 HCl 2 KCl + H 2 O + CO 2 45. 8 g ? g actual: 46. 3 g

B. Percent Yield K 2 CO 3 + 2 HCl 2 KCl + H 2 O + CO 2 45. 8 g ? g actual: 46. 3 g Theoretical Yield: 45. 8 g 1 mol 2 mol 74. 55 K 2 CO 3 KCl g KCl = 49. 4 138. 21 g 1 mol g KCl K 2 CO 3 KCl

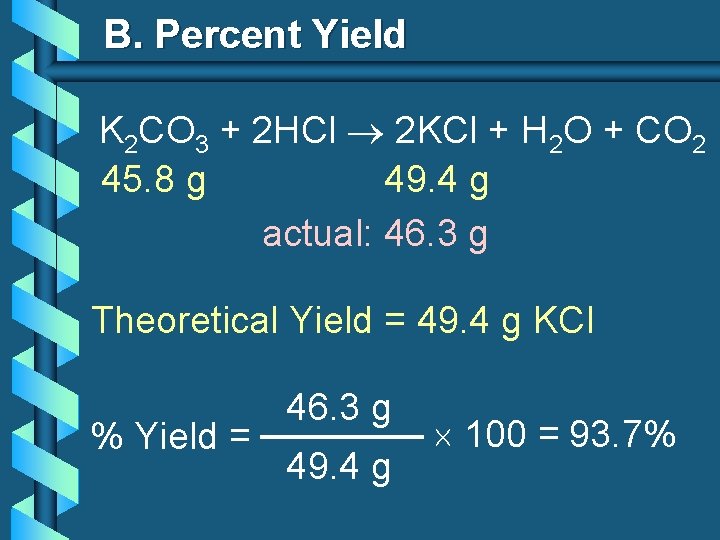
B. Percent Yield K 2 CO 3 + 2 HCl 2 KCl + H 2 O + CO 2 45. 8 g 49. 4 g actual: 46. 3 g Theoretical Yield = 49. 4 g KCl % Yield = 46. 3 g 49. 4 g 100 = 93. 7%
 Stoichiometry in the real world
Stoichiometry in the real world Real world stoichiometry
Real world stoichiometry Chó sói
Chó sói Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Thể thơ truyền thống
Thể thơ truyền thống Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập đại từ thay thế
đại từ thay thế Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi