Stoichiometry Ch 10 10 1 Stoichiometry Mass amt
















- Slides: 23

Stoichiometry Ch. 10

(10 -1) Stoichiometry • Mass & amt relationships b/w reactants & products – Conversions b/w grams & moles • Always begin w/ a balanced eq. !

Mass to Mol Reminder Convert 3. 5 g of Na. OH to mols Na. OH 1. List known 3. 5 g Na. OH 2. Calculate molar mass 22. 99 g/mol Na + 16 g/mol O + 1. 01 g/mol H = 40 g/mol 3. Set up problem & solve 3. 5 g Na. OH x 1 mol Na. OH = 0. 09 mol Na. OH 40 g Na. OH

Mol to Mass Reminder How many grams are in 6. 4 mols O 2? 1. List known 6. 4 mols O 2 2. Calculate molar mass (2) (16 g/mol O) = 32 g/mol O 2 3. Set up problem & solve 6. 4 mols O 2 x 32 g O 2 = 204. 8 g O 2 1 mol O 2

Mol to Mol Reminder If 2. 00 mol N 2 is reacting w/ a sufficient amt of H 2, how many mols of NH 3 will be produced? N 2 + H 2 NH 3 1. Balance the chemical eq. N 2 + 3 H 2 2 NH 3

Mol to Mol Reminder 2. Find the mole ratio 1 mol N 2 : 2 mol NH 3 3. Set up problem (begin w/ known) & solve 2. 00 mol N 2 x 2 mol NH 3 = 4 mol NH 3 1 mol N 2

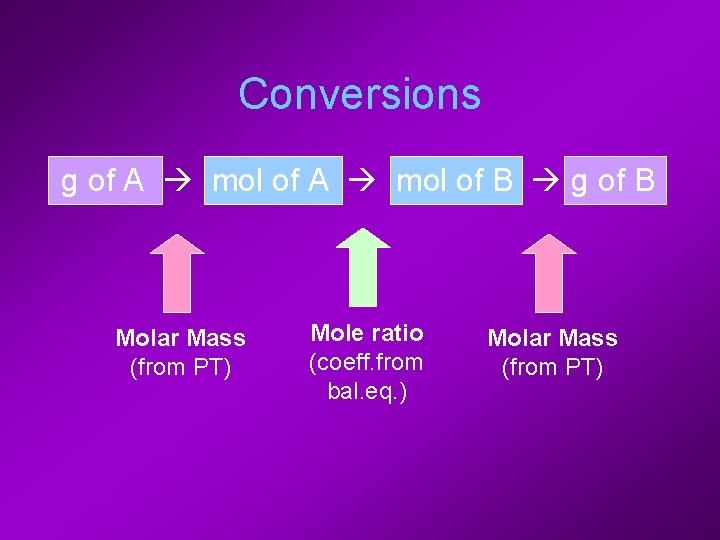
Conversions g of A mol of B g of B Molar Mass (from PT) Mole ratio (coeff. from bal. eq. ) Molar Mass (from PT)

Mass to Mass Practice How many grams of H 2 O are produced when 13 g O 2 combine w/ sufficient H 2? H 2 + O 2 H 2 O 1. Balance the chemical eq. 2 H 2 + O 2 2 H 2 O

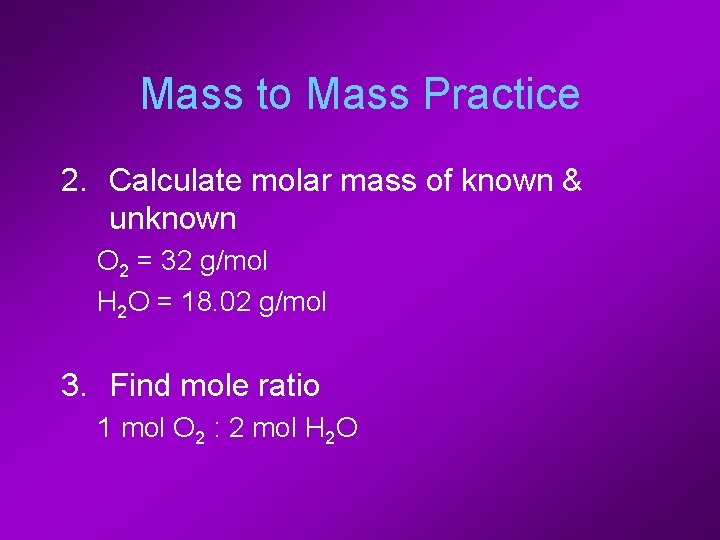
Mass to Mass Practice 2. Calculate molar mass of known & unknown O 2 = 32 g/mol H 2 O = 18. 02 g/mol 3. Find mole ratio 1 mol O 2 : 2 mol H 2 O

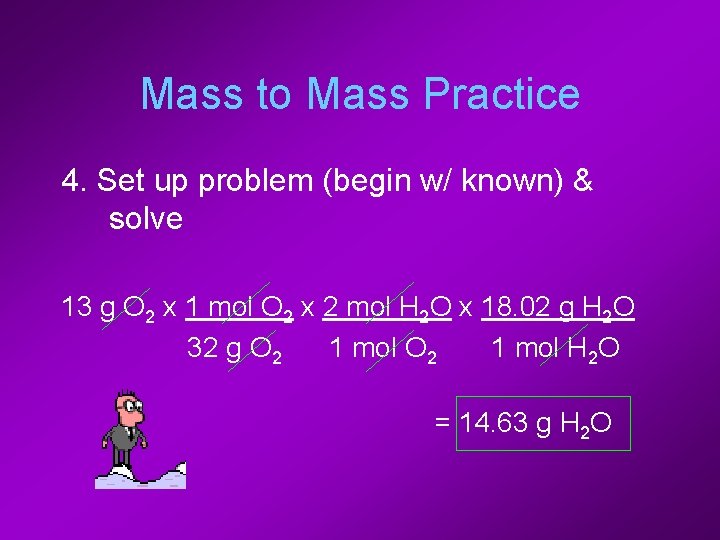
Mass to Mass Practice 4. Set up problem (begin w/ known) & solve 13 g O 2 x 1 mol O 2 x 2 mol H 2 O x 18. 02 g H 2 O 32 g O 2 1 mol H 2 O = 14. 63 g H 2 O

Using Density • Density (D) = mass (m) / volume (V) • Units: g/m. L • Convert from g m. L or m. L g

Density Practice Calculate the mass of Li. OH used to obtain 1500 m. L of water. (Hint: DH 2 O = 1. 00 g/m. L) CO 2 + 2 Li. OH Li 2 CO 3 + H 2 O 1. Start w/ known & use density to convert into grams 1500 m. L H 2 O x 1. 00 g H 2 O 1 m. L H 2 O

Density Practice 2. Proceed w/ mass to mass calc. 1500 m. L H 2 O x 1. 00 g H 2 O x 1 mol H 2 O x 1 m. L H 2 O 18. 02 g H 2 O 2 mol Li. OH x 23. 95 g Li. OH = 3989 g Li. OH 1 mol H 2 O 1 mol Li. OH

(10 -2) Excess Reactant • Extra left over after rxn • Limiting reactant: completely consumed – Limits amt of other reactants used – Determines max amt of product


Limiting Reactant Practice CO combines w/ H 2 to produce CH 3 OH. If you had 152. 5 g CO & 24. 5 g H 2 what mass of CH 3 OH could be produced? 1. Write a bal. eq. CO + 2 H 2 CH 3 OH

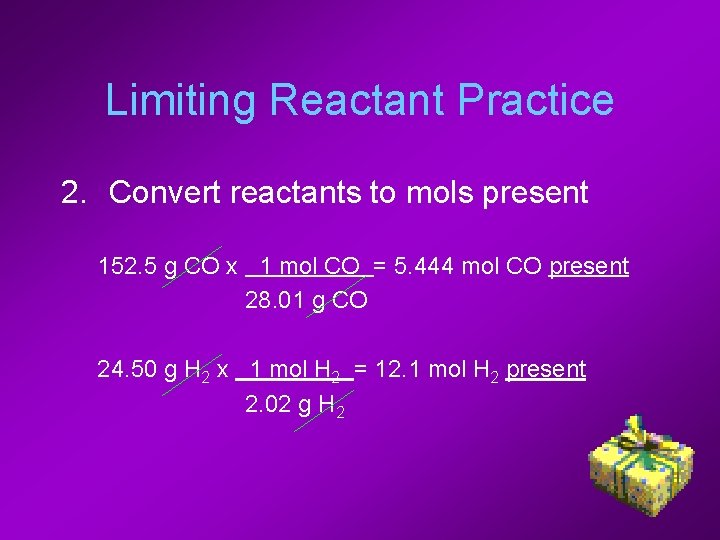
Limiting Reactant Practice 2. Convert reactants to mols present 152. 5 g CO x 1 mol CO = 5. 444 mol CO present 28. 01 g CO 24. 50 g H 2 x 1 mol H 2 = 12. 1 mol H 2 present 2. 02 g H 2

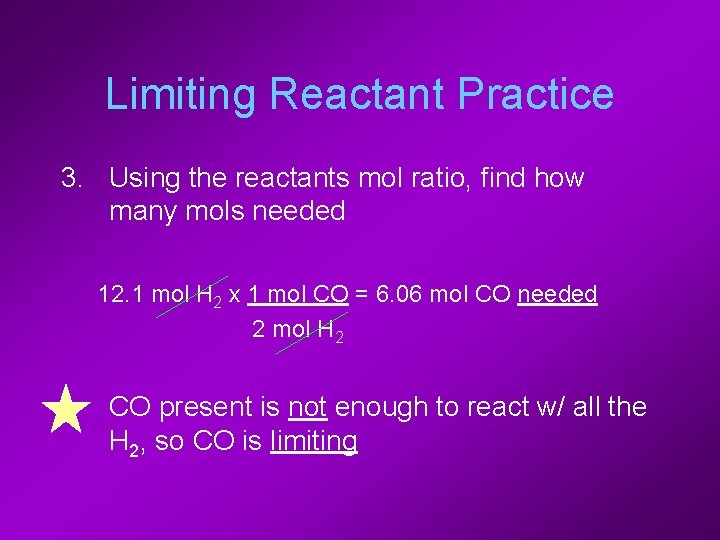
Limiting Reactant Practice 3. Using the reactants mol ratio, find how many mols needed 12. 1 mol H 2 x 1 mol CO = 6. 06 mol CO needed 2 mol H 2 • CO present is not enough to react w/ all the H 2, so CO is limiting

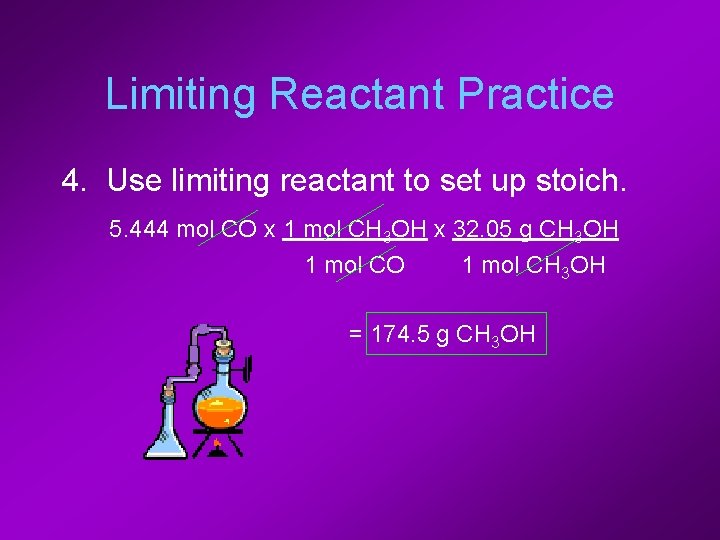
Limiting Reactant Practice 4. Use limiting reactant to set up stoich. 5. 444 mol CO x 1 mol CH 3 OH x 32. 05 g CH 3 OH 1 mol CO 1 mol CH 3 OH = 174. 5 g CH 3 OH

Theoretical Yield • Calculated max amt of product possible – Stoich. w/ limiting reactant – What should happen • Actual yield: measured amt of product experimentally produced – What does happen

Percentage Yield • How efficient a rxn is – How close actual is to theoretical – Should be 100% • % yield = actual x 100 theoretical

% Yield Practice When 0. 835 mol Li. OH is reacted w/ excess KCl, the actual yield of Li. Cl is 16 g. What is the % yield? Li. OH + KCl Li. Cl + KOH

% Yield Practice 1. Calculate theor. yield 0. 835 mol Li. OH x 1 mol Li. Cl x 42. 44 g Li. Cl = 35. 4 g Li. Cl 1 mol Li. OH 1 mol Li. Cl 2. Calculate the % yield = act. X 100 = 16 g Li. Cl x 100 = 45 % theor. 35. 4 g Li. Cl
 Ideal stoichiometric calculations
Ideal stoichiometric calculations Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Hgbp
Hgbp Amt für ernährung landwirtschaft und forsten rosenheim
Amt für ernährung landwirtschaft und forsten rosenheim Amt für kultur bozen
Amt für kultur bozen Amt für ernährung landwirtschaft und forsten rosenheim
Amt für ernährung landwirtschaft und forsten rosenheim Amt sport
Amt sport Amt chapter locations
Amt chapter locations Amt für umweltschutz uri
Amt für umweltschutz uri Amt setup prompt
Amt setup prompt Amt für soziale angelegenheiten trier
Amt für soziale angelegenheiten trier Amt sport
Amt sport Amt learning goals
Amt learning goals Intel amt inv
Intel amt inv Amt für regionale landesentwicklung weser-ems
Amt für regionale landesentwicklung weser-ems Lebensmittelkontrolle aargau
Lebensmittelkontrolle aargau Amt standards of practice
Amt standards of practice Amt für gesundheit st. gallen
Amt für gesundheit st. gallen Amt für raumentwicklung st. gallen
Amt für raumentwicklung st. gallen Assessorat für landwirtschaft
Assessorat für landwirtschaft Stoichiometry molar mass
Stoichiometry molar mass Gravitational mass vs inertial mass
Gravitational mass vs inertial mass The units of molar mass are
The units of molar mass are Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same