STOICHIOMETRY Calculations Based on Chemical Equations Iron III
STOICHIOMETRY Calculations Based on Chemical Equations
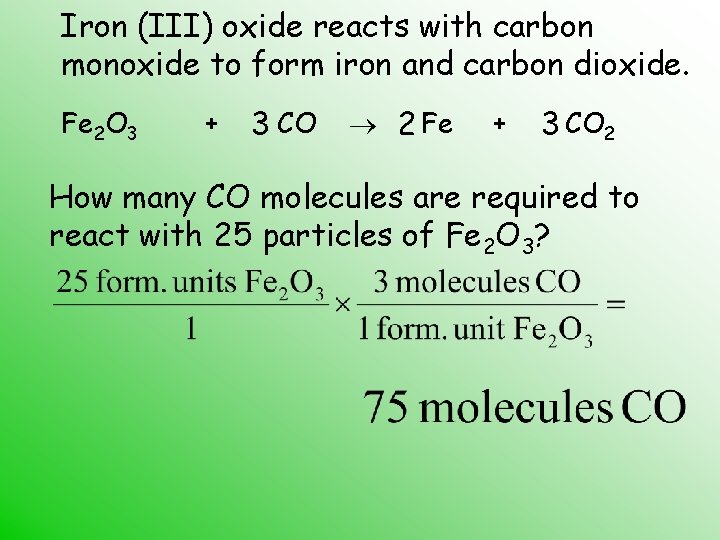
Iron (III) oxide reacts with carbon monoxide to form iron and carbon dioxide. Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 How many CO molecules are required to react with 25 particles of Fe 2 O 3?
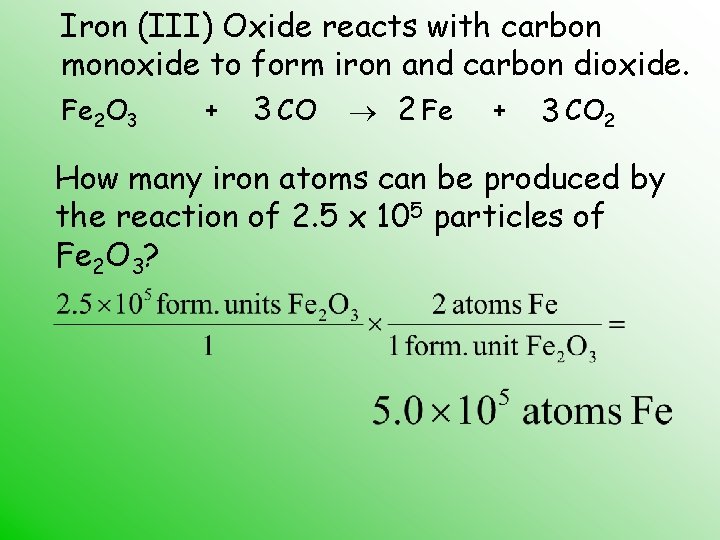
Iron (III) Oxide reacts with carbon monoxide to form iron and carbon dioxide. Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 How many iron atoms can be produced by the reaction of 2. 5 x 105 particles of Fe 2 O 3?
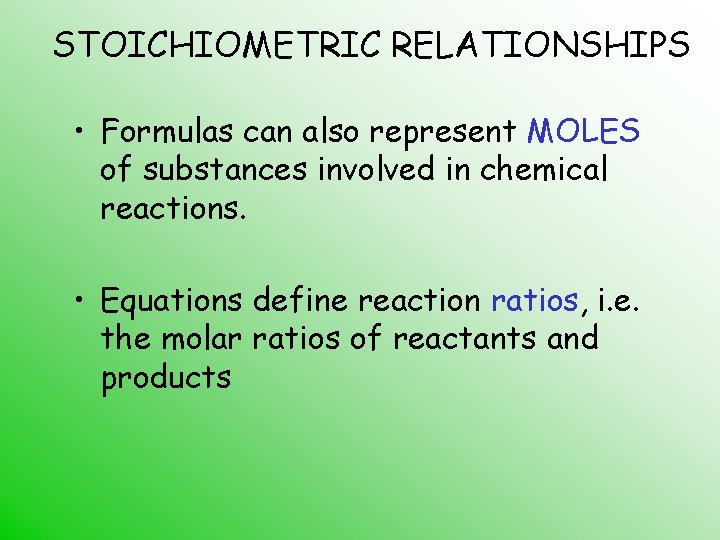
STOICHIOMETRIC RELATIONSHIPS • Formulas can also represent MOLES of substances involved in chemical reactions. • Equations define reaction ratios, i. e. the molar ratios of reactants and products
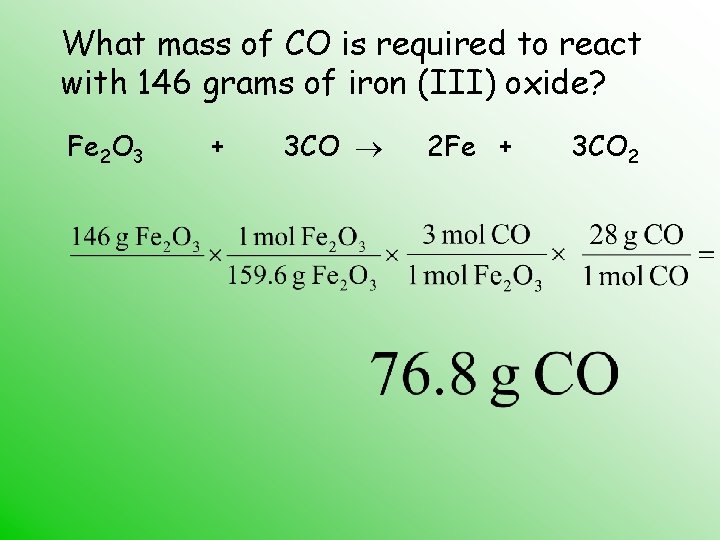
What mass of CO is required to react with 146 grams of iron (III) oxide? Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2
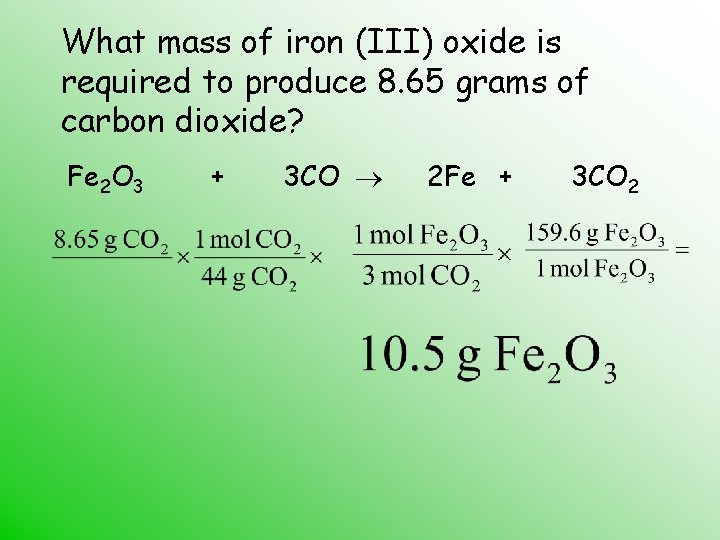
What mass of iron (III) oxide is required to produce 8. 65 grams of carbon dioxide? Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2

Limiting Reactants (Reagents) and Percent Yield

Calculations need to be based on the limiting reactant. • Example 1: Suppose a box contains 87 bolts, 110 washers and 99 nails. How many sets of 1 bolt, 2 washers and 1 nail can you use to create? What is the limiting factor? 55 sets; washers limit the amount

Calculations need to be based on the limiting reactant. • Example 2: What is the maximum mass of sulfur dioxide that can be produced by the reaction of 95. 6 g carbon disulfide with 100. g oxygen? CS 2 + O 2 CO 2 + SO 2 Start by balancing the equation…

Calculations need to be based on the limiting reactant. • Example 2: What is the maximum mass of sulfur dioxide that can be produced by the reaction of 95. 6 g carbon disulfide with 100. g oxygen? CS 2 + 3 O 2 CO 2 + 2 SO 2 Now solve the problem…
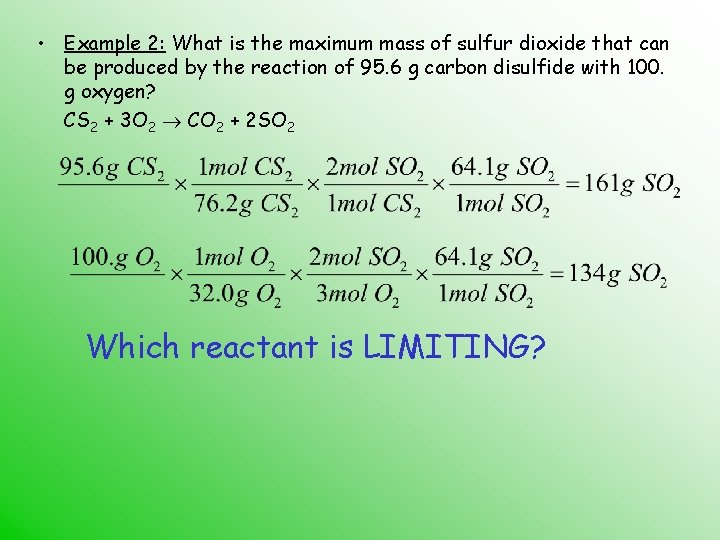
• Example 2: What is the maximum mass of sulfur dioxide that can be produced by the reaction of 95. 6 g carbon disulfide with 100. g oxygen? CS 2 + 3 O 2 CO 2 + 2 SO 2 Which reactant is LIMITING?
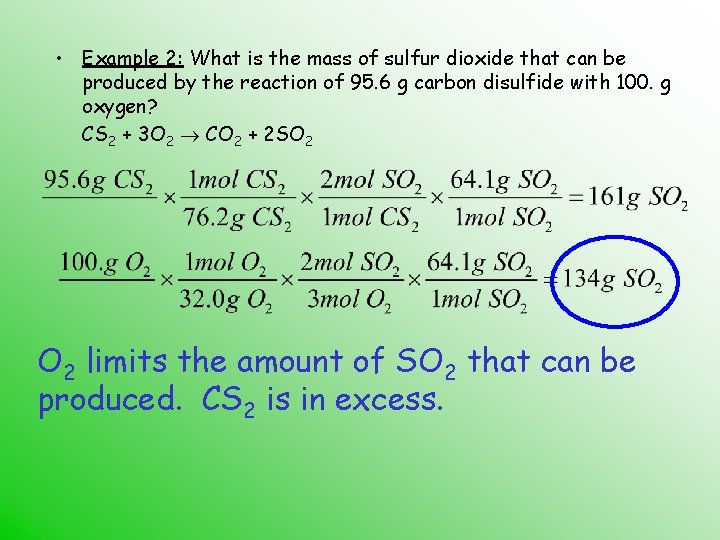
• Example 2: What is the mass of sulfur dioxide that can be produced by the reaction of 95. 6 g carbon disulfide with 100. g oxygen? CS 2 + 3 O 2 CO 2 + 2 SO 2 limits the amount of SO 2 that can be produced. CS 2 is in excess.
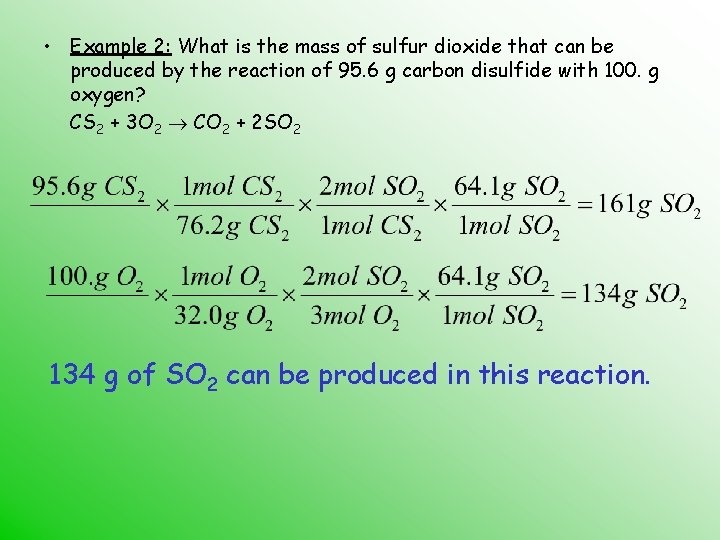
• Example 2: What is the mass of sulfur dioxide that can be produced by the reaction of 95. 6 g carbon disulfide with 100. g oxygen? CS 2 + 3 O 2 CO 2 + 2 SO 2 134 g of SO 2 can be produced in this reaction.
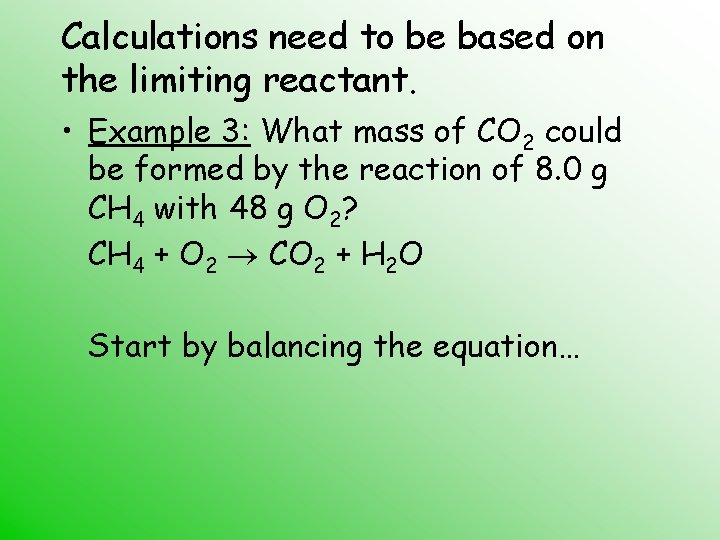
Calculations need to be based on the limiting reactant. • Example 3: What mass of CO 2 could be formed by the reaction of 8. 0 g CH 4 with 48 g O 2? CH 4 + O 2 CO 2 + H 2 O Start by balancing the equation…
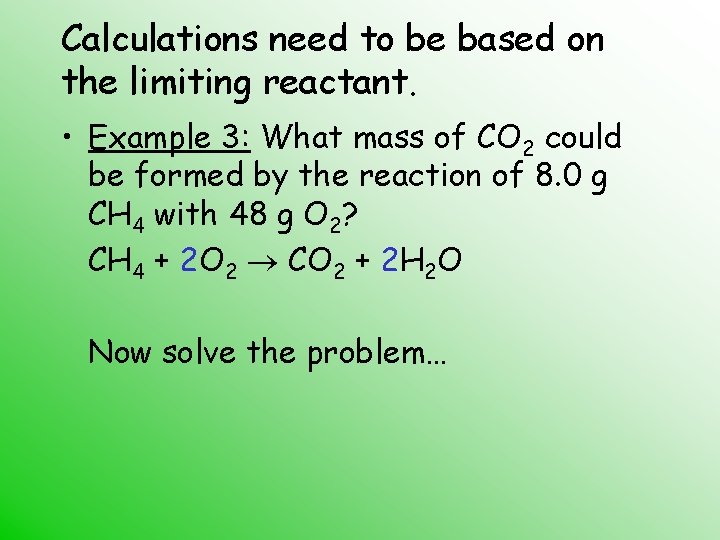
Calculations need to be based on the limiting reactant. • Example 3: What mass of CO 2 could be formed by the reaction of 8. 0 g CH 4 with 48 g O 2? CH 4 + 2 O 2 CO 2 + 2 H 2 O Now solve the problem…
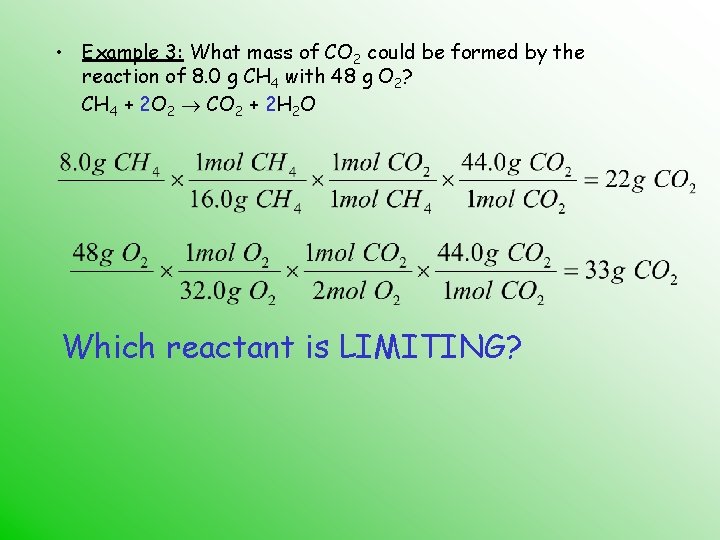
• Example 3: What mass of CO 2 could be formed by the reaction of 8. 0 g CH 4 with 48 g O 2? CH 4 + 2 O 2 CO 2 + 2 H 2 O Which reactant is LIMITING?
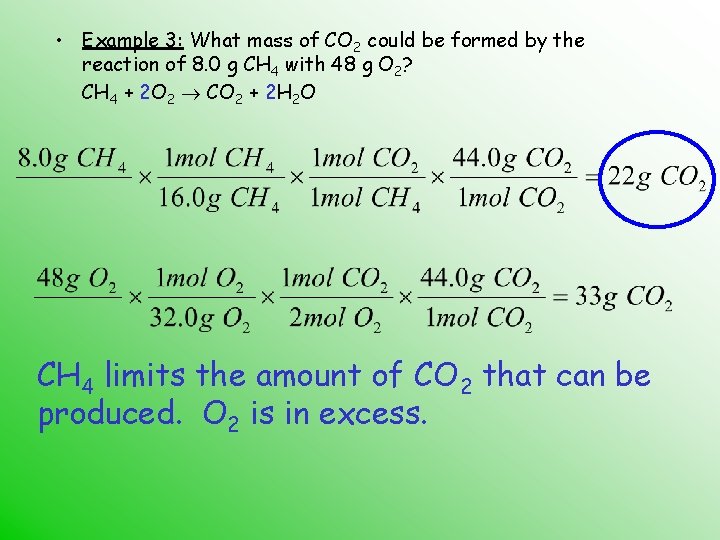
• Example 3: What mass of CO 2 could be formed by the reaction of 8. 0 g CH 4 with 48 g O 2? CH 4 + 2 O 2 CO 2 + 2 H 2 O CH 4 limits the amount of CO 2 that can be produced. O 2 is in excess.
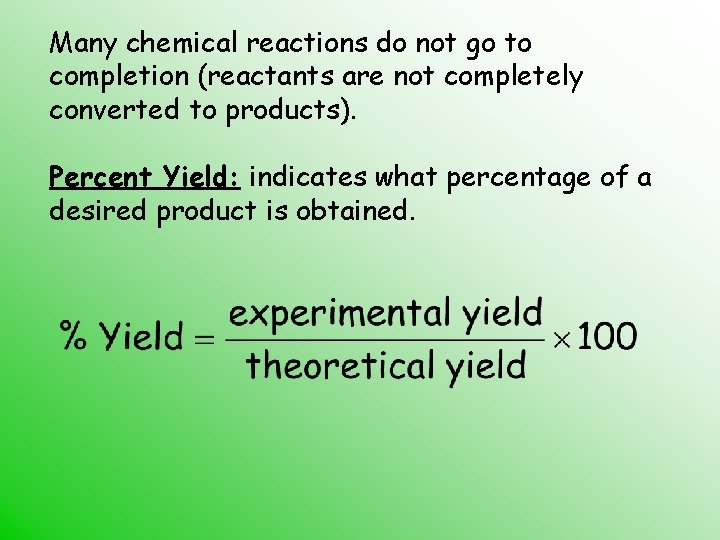
Many chemical reactions do not go to completion (reactants are not completely converted to products). Percent Yield: indicates what percentage of a desired product is obtained.
• So far, the masses we have calculated from chemical equations were based on the assumption that each reaction occurred 100%. • The THEORETICAL YIELD is the yield calculated from the balance equation. • The ACTUAL YIELD is the amount “actually” obtained in an experiment.
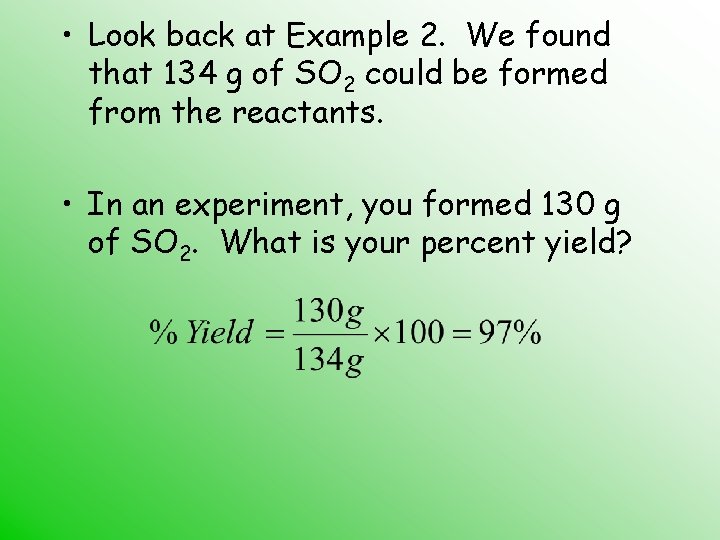
• Look back at Example 2. We found that 134 g of SO 2 could be formed from the reactants. • In an experiment, you formed 130 g of SO 2. What is your percent yield?
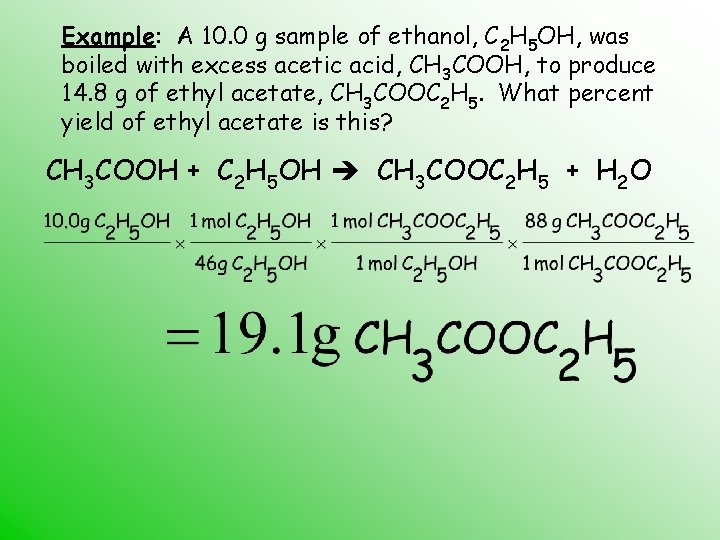
Example: A 10. 0 g sample of ethanol, C 2 H 5 OH, was boiled with excess acetic acid, CH 3 COOH, to produce 14. 8 g of ethyl acetate, CH 3 COOC 2 H 5. What percent yield of ethyl acetate is this? CH 3 COOH + C 2 H 5 OH CH 3 COOC 2 H 5 + H 2 O
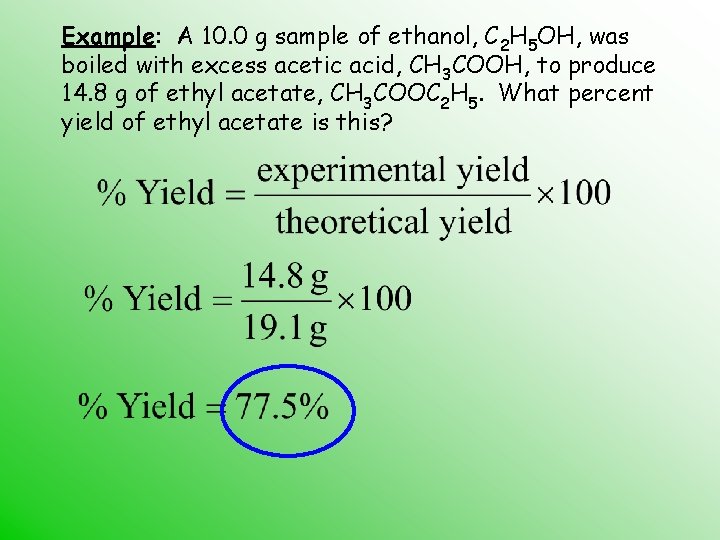
Example: A 10. 0 g sample of ethanol, C 2 H 5 OH, was boiled with excess acetic acid, CH 3 COOH, to produce 14. 8 g of ethyl acetate, CH 3 COOC 2 H 5. What percent yield of ethyl acetate is this?
- Slides: 22