STOICHIOMETRY AND THE COMBUSTION OF FUELS 2 CALCULATIONS
STOICHIOMETRY AND THE COMBUSTION OF FUELS 2 CALCULATIONS INVOLVING GASES AND FUELS
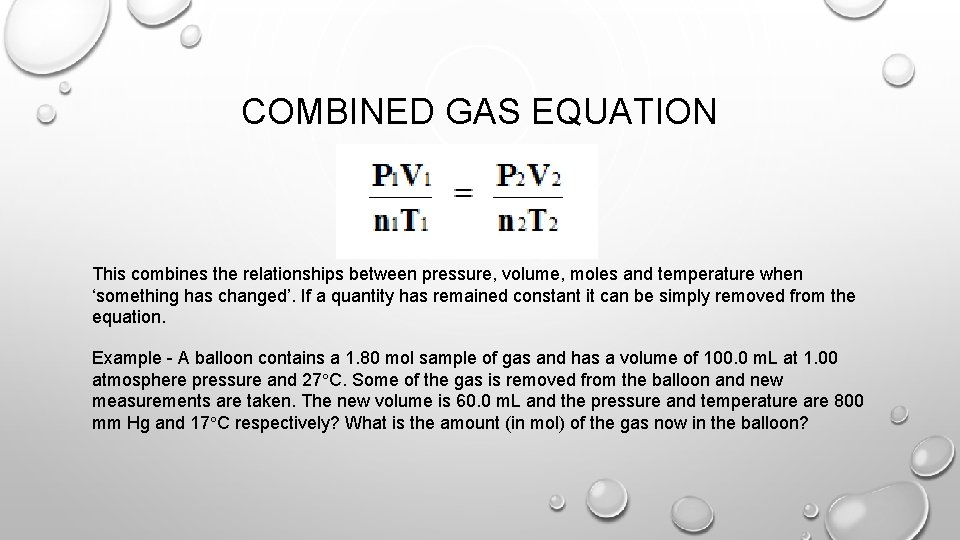
COMBINED GAS EQUATION This combines the relationships between pressure, volume, moles and temperature when ‘something has changed’. If a quantity has remained constant it can be simply removed from the equation. Example - A balloon contains a 1. 80 mol sample of gas and has a volume of 100. 0 m. L at 1. 00 atmosphere pressure and 27 C. Some of the gas is removed from the balloon and new measurements are taken. The new volume is 60. 0 m. L and the pressure and temperature are 800 mm Hg and 17 C respectively? What is the amount (in mol) of the gas now in the balloon?
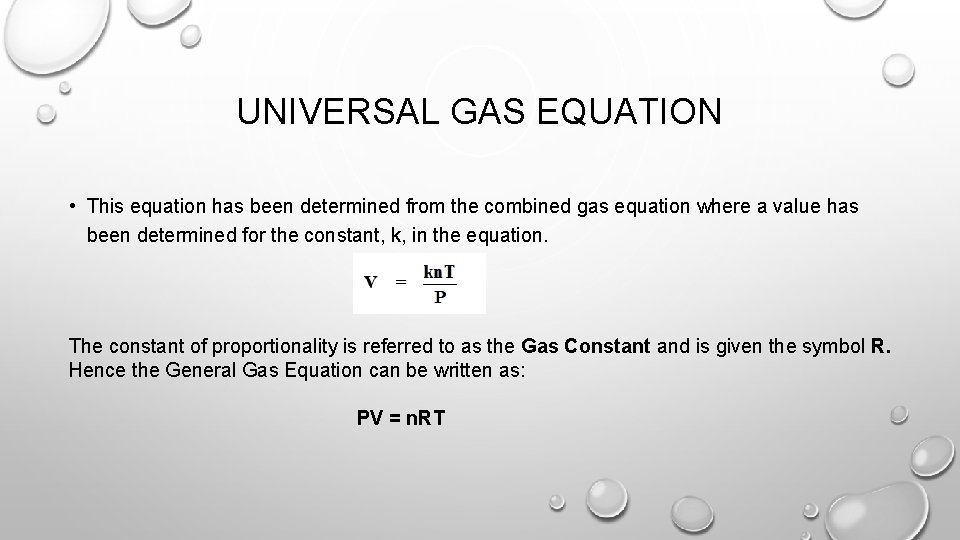
UNIVERSAL GAS EQUATION • This equation has been determined from the combined gas equation where a value has been determined for the constant, k, in the equation. The constant of proportionality is referred to as the Gas Constant and is given the symbol R. Hence the General Gas Equation can be written as: PV = n. RT
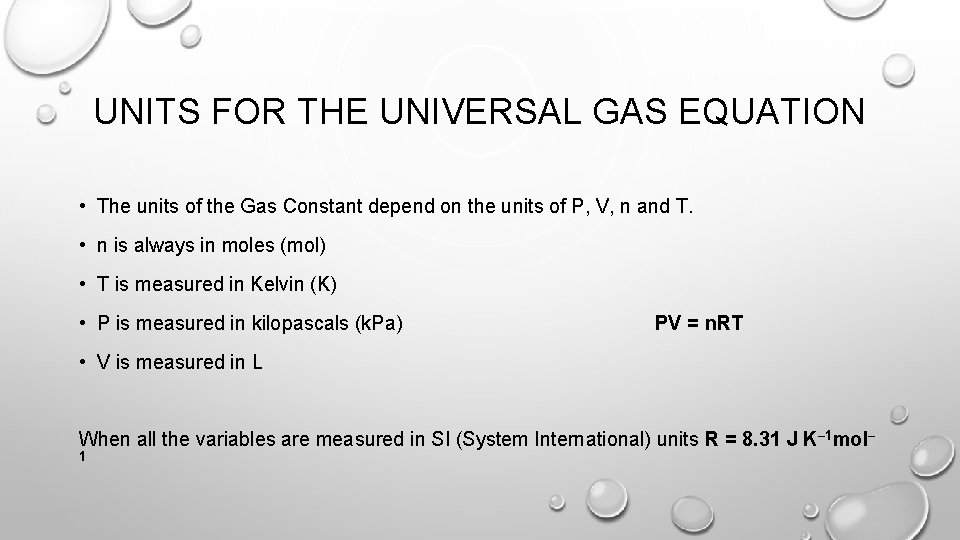
UNITS FOR THE UNIVERSAL GAS EQUATION • The units of the Gas Constant depend on the units of P, V, n and T. • n is always in moles (mol) • T is measured in Kelvin (K) • P is measured in kilopascals (k. Pa) PV = n. RT • V is measured in L When all the variables are measured in SI (System International) units R = 8. 31 J K– 1 mol– 1

Using the universal gas equation If 0. 107 mol of a gas occupies 2000 m. L at 0. 500 atm, what is its temperature in o. C? Try q 5 -8 on p 80 of your text
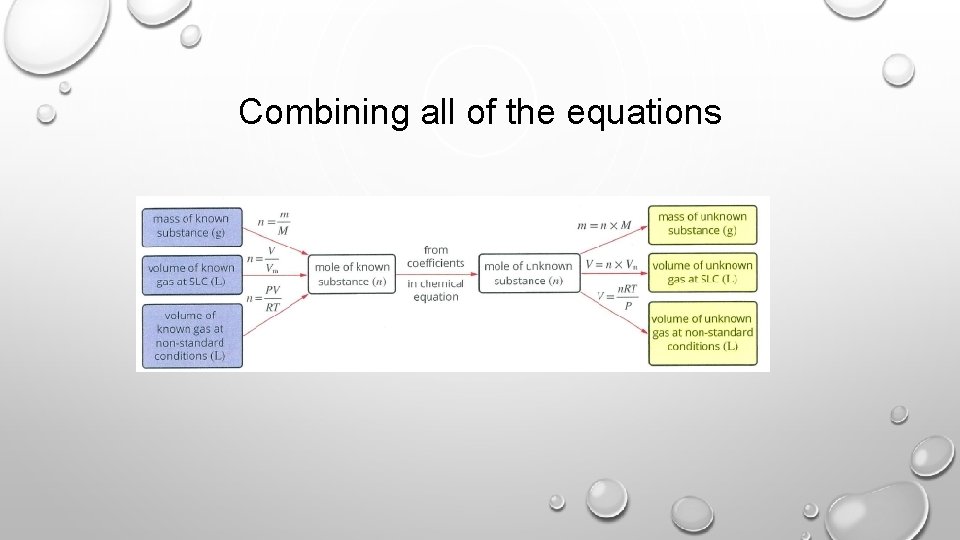
Combining all of the equations
Mass-Mass stoichiometric problems Calculate the mass of carbon dioxide produced when 3. 60 kg of butane (C 4 H 10) is burned completely in oxygen 1. Write the equation and balance it 2. Determine the moles of the known quantity 3. Use the molar ratio in the equation to determine the moles of the unknown quantity 4. Use the moles of the unknown substance to determine the mass P 84 – complete q 1 -5
Mass – Volume stoichiometric problems • Connecting mass and volume again involves moles but this time temperature is important as it will determine whether Vm or PV = n. RT is used. Example 1 (mass volume – standard) Calculate the volume of CO 2, in L, produced when 300 g of butane is burned completely in oxygen. The gas is measured at SLC.

Example 2 – mass volume (non standard) • Calculate the volume of CO 2, in L, produced when 5. 00 kg of butane is burned completely in oxygen. The gas is measured at 40˚C and 400 k. Pa.

Example 3 – Volume-volume • If all reactants are gases then the volumes can act as the molar ratios • Methane gas (CH 4) is burned un a gas stove according to the following equation CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) • If 50 m. L of methane is burned in air, calculate the volume of CO 2 gas produced under constant temperature and pressure P 90 – complete q 1 -5
- Slides: 10