Stock SystemNew Naming System Some metal elements can











- Slides: 30

Stock System-New Naming System • Some metal elements can form two or more cations with a different charge. The stock system denotes the specific ion by putting the charge in ( ). • Example: • Iron (II) has a + 2 charge • Iron (III) has a +3 charge

Other common examples • • • Lead (II) has a +2 charge Lead (IV) has a +4 charge Tin (II) has a + 2 charge Tin (IV) has a +4 charge Copper (I) has a +1 charge Copper (II) has a +2 charge

Old Nomenclature-Don’t have to memorize these • • Copper (I) – cuprous Copper (II) – cupric Iron (II) – ferrous Iron (III) – ferric Tin (II) – stannous Tin (IV) – stannic Lead (II) – plumbous Lead (IV) – plumbic

Naming ionic compounds • Nomenclature-naming system – Positive ions is first, then negative ion – Ex: sodium chloride Na. Cl

Practice • Write formulas for the following: • Copper (II) Sulfate – Cu. SO 4 • Iron (III) oxide – Fe 2 O 3 • Zinc (II) sulfide – Zn. S • Vandium (IV) chloride – VCl 4 • Cobalt (III) Nitrite – Co(NO 3)3

Practice • http: //www. quia. com/jg/65800. html -

Compounds containing Polyatomic ions • Treat the polyatomic ion as a single atom • Always put polyatomic ion in parentheses if there is more than 1 of them • Example: ammonium chloride – NH 4 Cl Practice • Barium Nitrate – Ba(NO 3)2 • Fe. SO 4 – iron (II) sulfate

Naming Molecular Compounds • Old way of naming Molecular Compounds requires the Use of prefixes. (memorize These) 1 = mono 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca

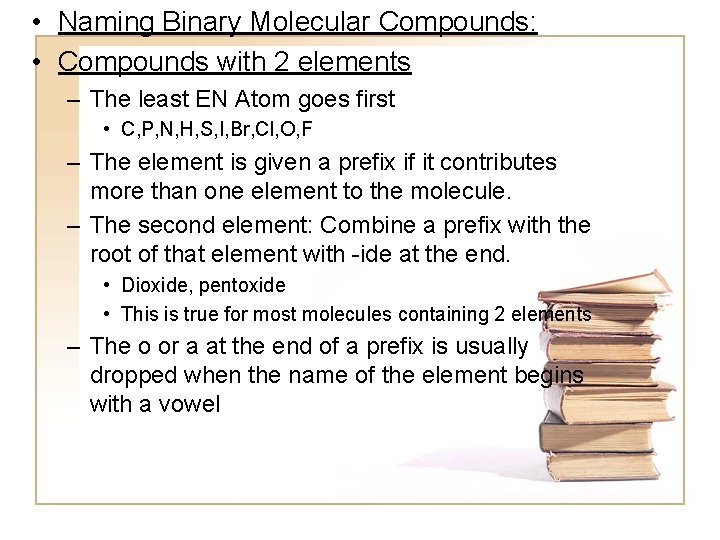
• Naming Binary Molecular Compounds: • Compounds with 2 elements – The least EN Atom goes first • C, P, N, H, S, I, Br, Cl, O, F – The element is given a prefix if it contributes more than one element to the molecule. – The second element: Combine a prefix with the root of that element with -ide at the end. • Dioxide, pentoxide • This is true for most molecules containing 2 elements – The o or a at the end of a prefix is usually dropped when the name of the element begins with a vowel

Practice 1. CO – Carbon Monoxide 2. CO 2 – Carbon Dioxide 3. CCl 4 – Carbon Tetra. Chloride 4. Si. F 4– Silicon Tetraflouride 5. Nitrogen Monoxide – NO 6. N 2 O 3 – Dinitrogen Trioxide 7. Phosphorus Trichloride – PCl 3

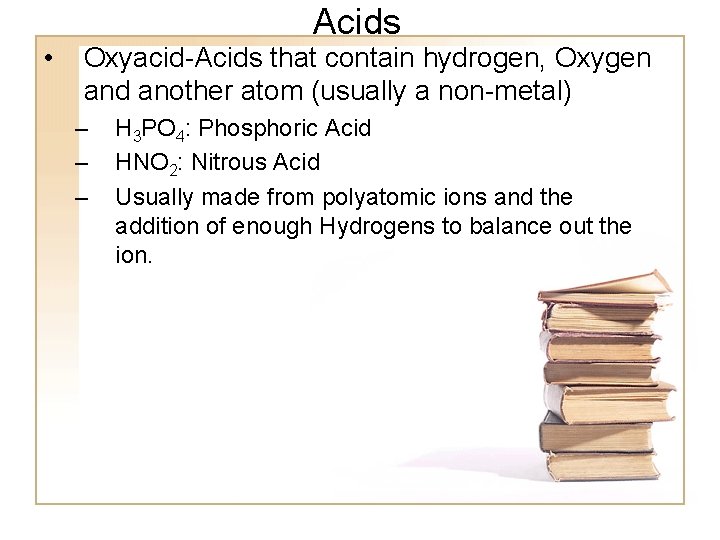
• Acids Oxyacid-Acids that contain hydrogen, Oxygen and another atom (usually a non-metal) – – – H 3 PO 4: Phosphoric Acid HNO 2: Nitrous Acid Usually made from polyatomic ions and the addition of enough Hydrogens to balance out the ion.

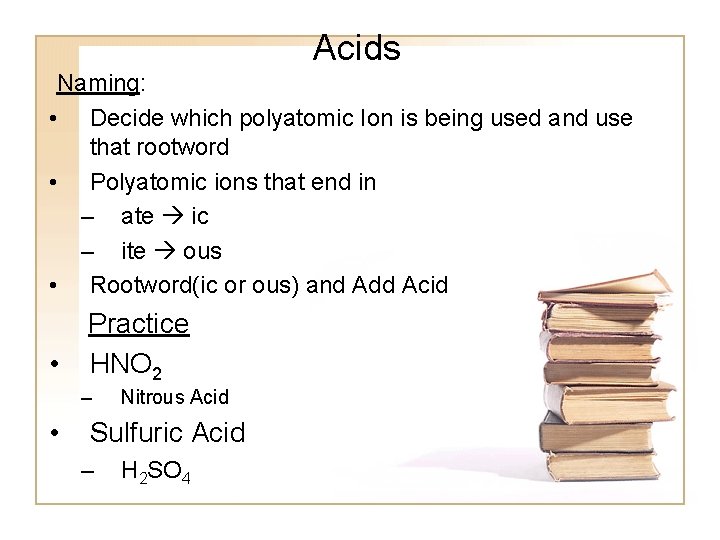
Acids Naming: • Decide which polyatomic Ion is being used and use that rootword • Polyatomic ions that end in – ate ic – ite ous • Rootword(ic or ous) and Add Acid • Practice HNO 2 – • Nitrous Acid Sulfuric Acid – H 2 SO 4

Acids • Binary Acids-Acids that consist of 2 elements: usually Hydrogen and one of the Halogens (there are others) – – • • • Naming: Hydro goes first Add the name of the second atom with ic at the end H 2 S – • HCl: Hydrochloric Acid HF: Hydroflouric Acid Hydrosulfuric Acid Hydrobromic Acid – HBr

Acids-Practice • HI – • CH 3 COOH – • Acetic Acid Sufurous Acid – • • Hydroiodic Acid H 2 SO 3 Carbonic Acid H 2 CO 3

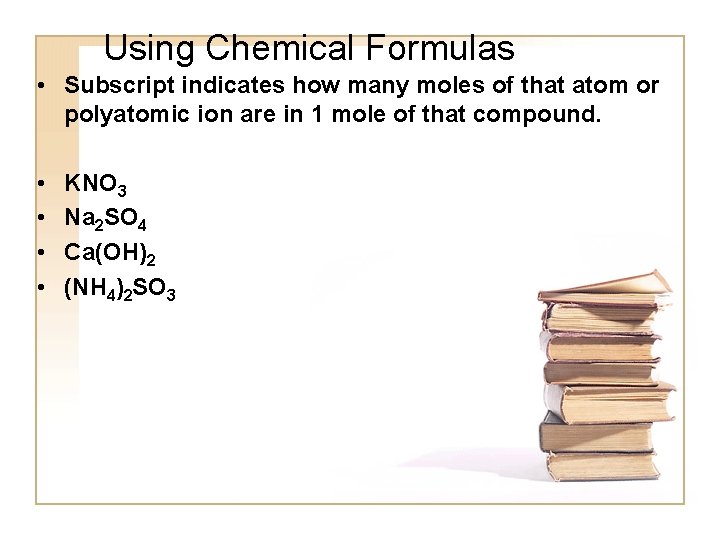
Using Chemical Formulas • Subscript indicates how many moles of that atom or polyatomic ion are in 1 mole of that compound. • • KNO 3 Na 2 SO 4 Ca(OH)2 (NH 4)2 SO 3

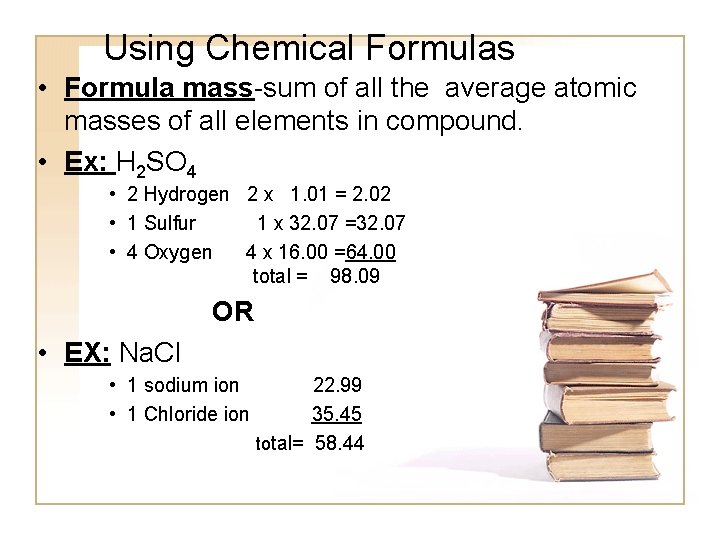
Using Chemical Formulas • Formula mass-sum of all the average atomic masses of all elements in compound. • Ex: H 2 SO 4 • 2 Hydrogen 2 x 1. 01 = 2. 02 • 1 Sulfur 1 x 32. 07 =32. 07 • 4 Oxygen 4 x 16. 00 =64. 00 total = 98. 09 OR • EX: Na. Cl • 1 sodium ion • 1 Chloride ion 22. 99 35. 45 total= 58. 44

Practice • (NH 4)3(PO 4) • Al 2 O 3 • Na 2 O

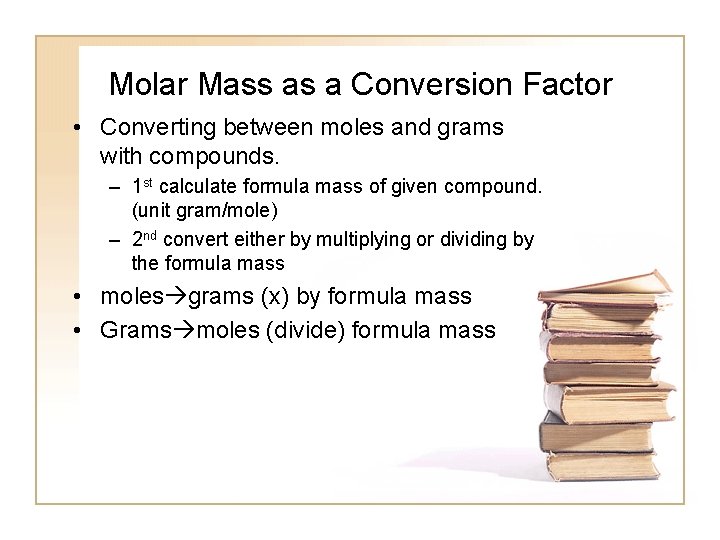
Molar Mass as a Conversion Factor • Converting between moles and grams with compounds. – 1 st calculate formula mass of given compound. (unit gram/mole) – 2 nd convert either by multiplying or dividing by the formula mass • moles grams (x) by formula mass • Grams moles (divide) formula mass

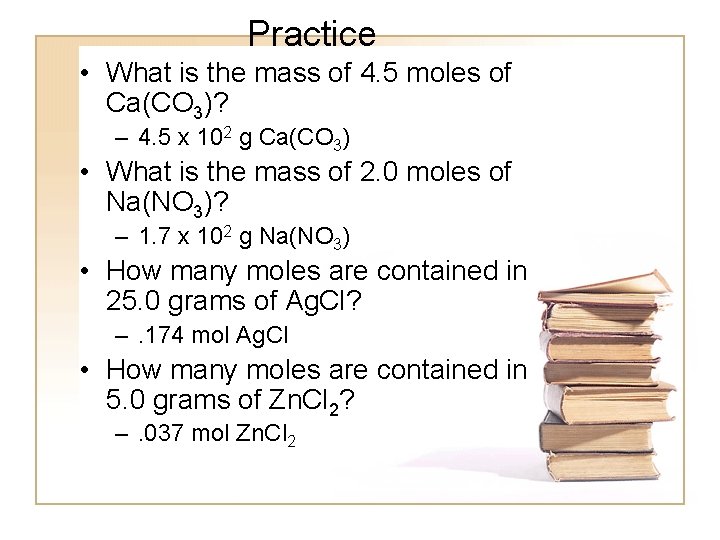
Practice • What is the mass of 4. 5 moles of Ca(CO 3)? – 4. 5 x 102 g Ca(CO 3) • What is the mass of 2. 0 moles of Na(NO 3)? – 1. 7 x 102 g Na(NO 3) • How many moles are contained in 25. 0 grams of Ag. Cl? –. 174 mol Ag. Cl • How many moles are contained in 5. 0 grams of Zn. Cl 2? –. 037 mol Zn. Cl 2

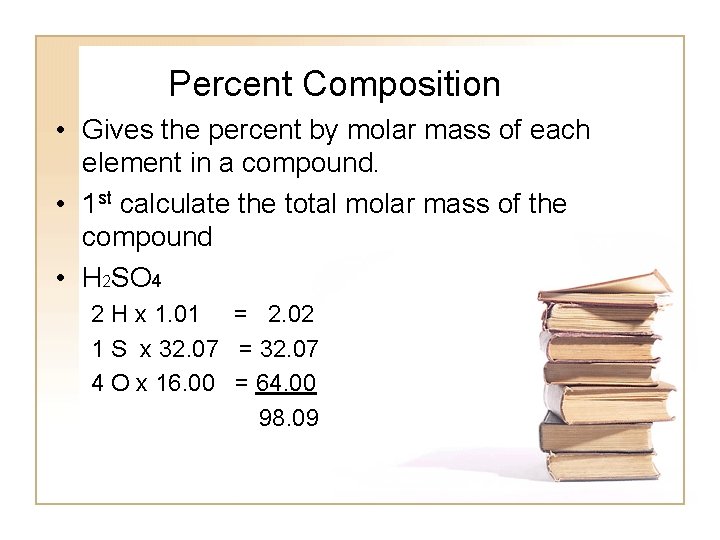
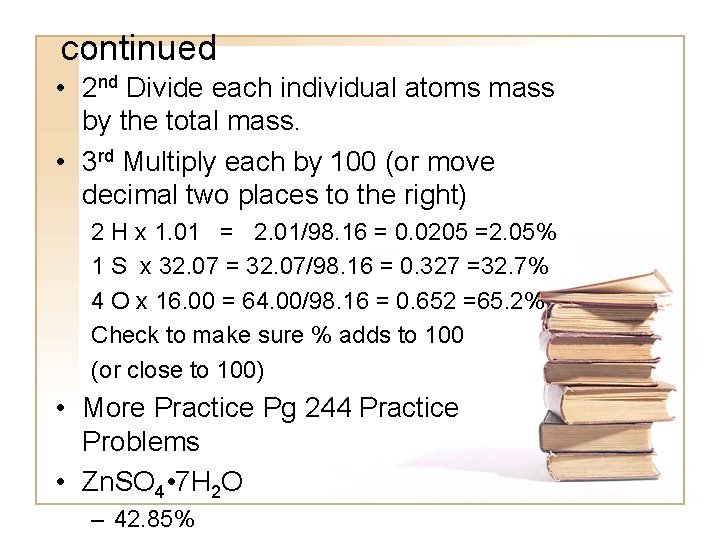
Percent Composition • Gives the percent by molar mass of each element in a compound. • 1 st calculate the total molar mass of the compound • H 2 SO 4 2 H x 1. 01 = 2. 02 1 S x 32. 07 = 32. 07 4 O x 16. 00 = 64. 00 98. 09

continued • 2 nd Divide each individual atoms mass by the total mass. • 3 rd Multiply each by 100 (or move decimal two places to the right) 2 H x 1. 01 = 2. 01/98. 16 = 0. 0205 =2. 05% 1 S x 32. 07 = 32. 07/98. 16 = 0. 327 =32. 7% 4 O x 16. 00 = 64. 00/98. 16 = 0. 652 =65. 2% Check to make sure % adds to 100 (or close to 100) • More Practice Pg 244 Practice Problems • Zn. SO 4 • 7 H 2 O – 42. 85%

Determining Chemical Formulas • Empirical formula-symbols of elements in compound showing the smallest whole number ratio. • Convert percents of elements in a compound into formula.

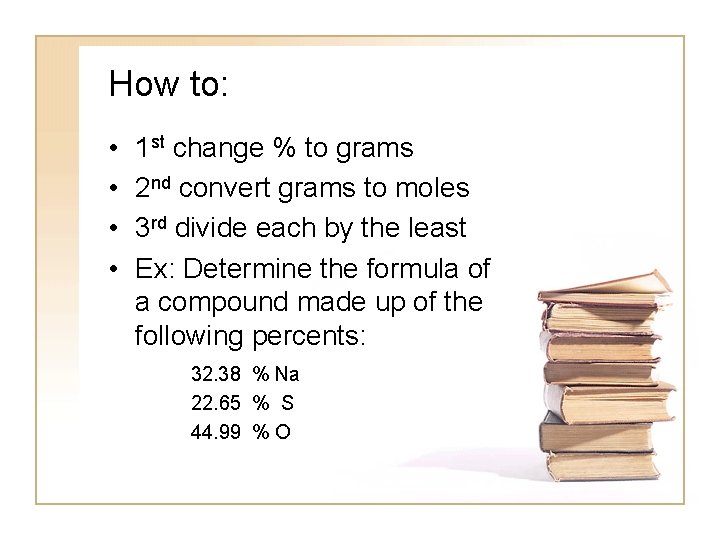
How to: • • 1 st change % to grams 2 nd convert grams to moles 3 rd divide each by the least Ex: Determine the formula of a compound made up of the following percents: 32. 38 % Na 22. 65 % S 44. 99 % O

Practice problems 1 -3 p. 247

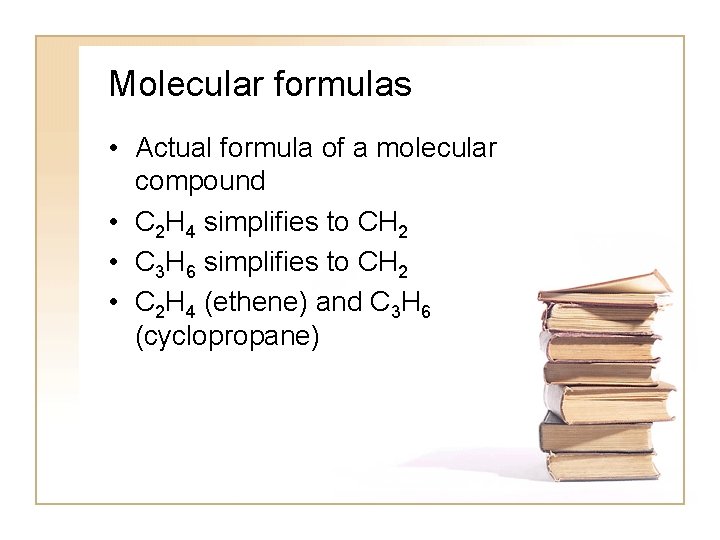
Molecular formulas • Actual formula of a molecular compound • C 2 H 4 simplifies to CH 2 • C 3 H 6 simplifies to CH 2 • C 2 H 4 (ethene) and C 3 H 6 (cyclopropane)

How the empirical formula relates to the molecular formula: • • • EF = empirical formula MF = molecular formula X( EF) = (MF) 1 st solve for x X= (MF)/(EF) HINT-BIG NUMBER ALWAYS GOES ON TOP • Multiply (EF) by x to get the molecular formula

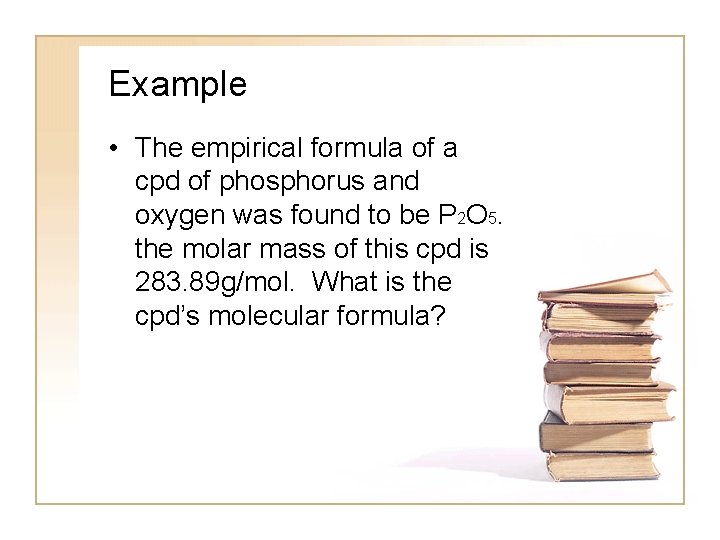
Example • The empirical formula of a cpd of phosphorus and oxygen was found to be P 2 O 5. the molar mass of this cpd is 283. 89 g/mol. What is the cpd’s molecular formula?

Practice Problems 1 -2 p. 249

Give the formula for the following: • • • 1. potassium oxide 2. lithium phosphide 3. aluminum chloride 4. calcium bromide 5. iron(II)oxide 6. iron(III)oxide 7. tin(IV)fluoride 8. copper(II)chloride 9. lead (II) sulfide 10. magnesium chloride

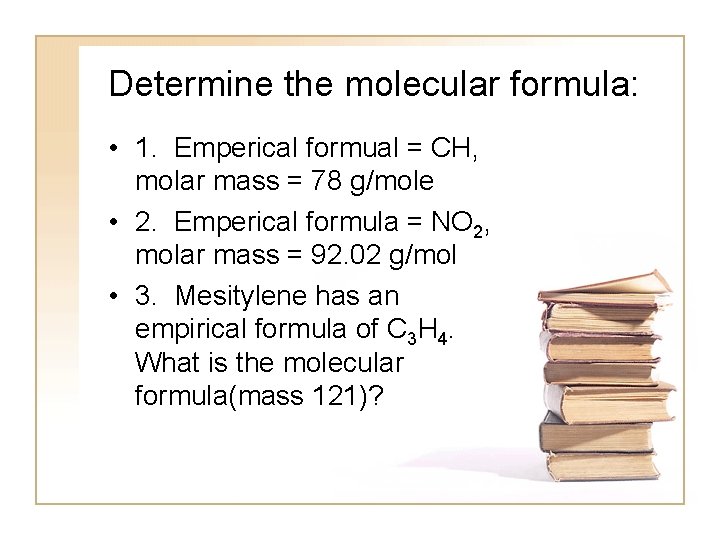
Determine the molecular formula: • 1. Emperical formual = CH, molar mass = 78 g/mole • 2. Emperical formula = NO 2, molar mass = 92. 02 g/mol • 3. Mesitylene has an empirical formula of C 3 H 4. What is the molecular formula(mass 121)?