STERILIZATION Definitions Sterilization Process by which an article

STERILIZATION
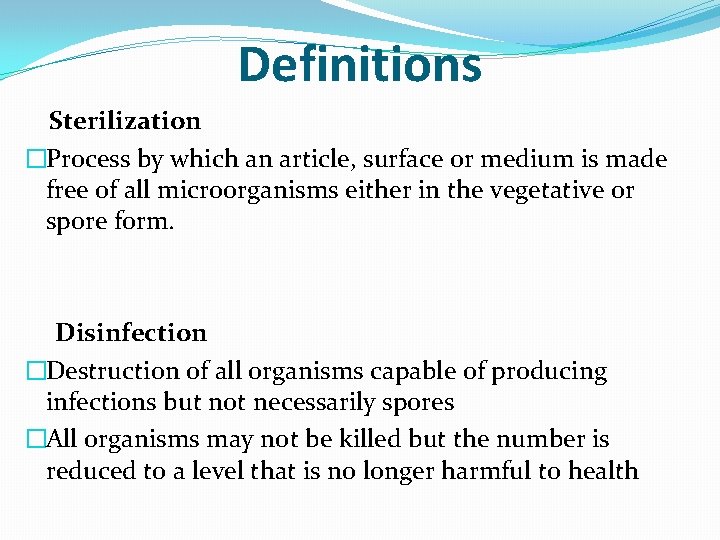
Definitions Sterilization �Process by which an article, surface or medium is made free of all microorganisms either in the vegetative or spore form. Disinfection �Destruction of all organisms capable of producing infections but not necessarily spores �All organisms may not be killed but the number is reduced to a level that is no longer harmful to health

�Antiseptics: �These are chemical disinfectants which can be safely applied to living tissues and are used to prevent infection by inhibiting growth of microorganisms �Asepsis: �The technique by which, the occurrence of infection into an uninfected tissue is prevented

Uses �For materials and instruments used in surgical and diagnostic procedures. �For media and reagents used in microbiology labaratory �In food and drug manufacturing to ensure safety from contaminating organisms

CLASSIFICATION Broadly classified into: 1) Physical methods. 2) Chemical methods. 3) Mechanical methods. Physical methods Drying Dry heat Moist heat Ionising Flaming Incineration Radiation Non-ionising Hot air oven Temperatures below Temperature at 1000 C Steam at Atmospheric Pressure (1000 C) Steam under pressure

CLASSIFICATION Sterilization Methods Physical Chemical

Classification Physical methods Sunlight Dry Red heat Flaming Incineration Hot air oven Heat Filtration Moist <100 C =100 C >100 C Radiation -Ionising -Non-ionising

Physical Methods �Sunlight �Heat – Dry – Red heat Flaming Incineration Hot air oven Moist – at temperature below 100 C at temperature of 100 C at temperature above 100 C �Filtration �Radiation

Chemical Methods �Alcohols ethyl alcohol, isopropyl alcohol �Aldehydes formaldehyde, glutaraldehyde �Phenols cresol, chlorhexidine �Halogens chlorine, iodine �Oxidizing agents hydrogen peroxide �Salts salts of copper, silver, mercury �Surface active agents cetrimide �Dyes crystal violet, acriflavine �Vapour phase disinfectants formaldehyde gas

�Mechanical methods: �Filtration �Ultrasonic vibration

PHYSICAL METHODS SUNLIGHT �Germicidal – UV rays. �Natural method – for water in tanks, rivers, lakes.

HEAT �Most reliable, commonly used �Two types – Dry heat and Moist heat �Principle �Factors effecting – Nature Temperature, duration Microorganisms and spores Material type

Dry Heat Sterilization �Principle : Denaturation of bacterial proteins, oxidative damage, toxic effect of elevated levels of electrolytes, DNA damage. �Methods : Red heat Flaming Incineration Hot air oven

Dry Heat Sterilization �Red heat – inoculation loops and wires, forceps tips, needles are held to the flame of bunsen burner till they become red hot �Flaming – glass slides, scalpels, mouths of culture tubes are passed through a bunsen flame, without allowing to become red hot �Incineration – using incinerator soiled dressings, animal carcasses, bedding, pathological materials are burnt to ashes inside the incinerator

Hot Air Oven �Electrically heated �Fan – adequate, even distribution of hot air �Thermostat – maintaining the chamber air at chosen temperature �Temperature and time – 160 C for a holding time of 2 hours (previously 1 hour)

Hot Air Oven

�Uses – 1)Glasswares - glass syringes, petridishes, flasks. 2) Surgical instruments -scalpels, scissors, forceps. 3) Chemicals – liquid paraffin �Sterilization control – o Spores of Bacillus subtilis subsp. niger or nontoxigenic strain of Clostridium tetani o Browne’s tube- after sterilisation green colour is produced

�Precautions : o No overloading. o Arrangement of materials should allow free air circulation o Materials should be completely dry o Use of cotton plugs to close test tubes, flasks o Paper wrapping of petri-plates, pipettes o No rubber, inflammable materials o Oven to be left for cooling for 2 hours before opening the door

�Disadvantage : o Hot air is a bad conductor of heat and its penetrating power is low

Moist Heat Sterilization Under 3 conditions : �At a temperature below 100 C. �At a temperature of 100 C. �At a temperature above 100 C. �Principle : denaturation and coagulation of proteins.

At a temperature below 100 C �Pasteurization : 2 types – Holder method – 63 C for 30 minutes. Flash method – 72 C for 20 secs, cooling to 13 C. �Inspissation : Using inspissator. For serum or egg media. 80 -85 C for ½ an hour. 3 consecutive days.

�Vaccine bath : Bacterial vaccines. 60 C for 1 hr. �Low temperature steam formaldehyde : Steam at subatmospheric pressure, temperature of 75 C, using formaldehyde vapour.

At a temperature of 100 C �Boiling : 10 -30 mins. Glass syringes, rubber stoppers. Not recommended for surgical instruments. �Tyndallisation : steam at 100 C for 20 mins for 3 days. Principle egg, serum, sugar containing media.

�Steam sterilization : 100 C for 90 mins at atmospheric pressure. Koch’s or Arnold’s steam steriliser. For media which cannot withstand high temperature. Vegetative forms killed.

At a temperature above 100 C Principle : �Water boils when vapour pressure becomes equal to atmospheric pressure. �Increase in atmospheric pressure – Increase in boiling temperature. �Normal pressure – water boils at 100 C. �Increase in pressure inside a closed vessel, increase in boiling temperature of water. �Used in autoclaves, pressure cookers.

Autoclave : Principle : �Saturated steam – better killing power, penetrate porous materials easily. �Steam on contact with cooler surfaces – condenses to water – liberation of latent heat. �Reduction in volume – more steam sucked into the same site. �Process continues till temperature of the article equals steam temperature. �Condensed water – moist conditions for killing microbes.

Components : �Vertical or horizontal cylinder in a supporting iron case. �Lid with screw clamps. �Discharge tap, pressure gauge, safety valve. �Heating with electricity. �Steam with high pressure supplied into the inner chamber.

Procedure : �Cylinder filled with water �Materials kept on the perforated tray. �Lid closed, discharge tap open �Heater put on. �Safety valve adjusted to required pressure. �Water allowed to boil. �Steam and air mixture allowed to escape. �Discharge tap closed.

�Steam pressure rises inside. �Desired level reached, safety valve opens for excess steam to escape. �Holding period counted for 15 minutes. �Heating stopped. �Autoclave allowed to cool. �Discharge tap opened slowly. �Lid is opened. �Sterilized materials removed.

Autoclave

�Sterilization conditions : 121 C for 15 mins at 15 pounds per square inch. �Uses : sterilize culture media, rubber material, gowns, dressings, gloves, etc. �Precautions : Complete removal of air from chamber. Proper arrangement of materials.

�Sterilization control : Thermocouple Bacterial spores Chemical indicators Autoclave tapes

FILTRATION �For substances which are damaged by heat. �Uses : Sterilize sera, sugars, antibiotic solutions. Separation of toxins, bacteriophages from bacteria. Obtaining bacteria free filtrates. Sterilize hydatid fluid. Diagnosis of causative agents of disease. Purification of water. Limitations : Filter pores retain bacteria but viruses pass through.

Types : �Candle filters : Purification of water. Form of hollow candles. eg : Berkefeld filters, Chamberland filters. • Asbestos disc filters : made of asbestos. eg: Seitz filter.


�Sintered glass filters : prepared by fusing finely powdered glass particles. �Membrane filters : made of cellulose esters. Used in water analysis, sterility testing. Widely used : nitrocellulose filters. Pore sizes vary from 0. 015 – 12 µm. 0. 22 µm filter widely used.


�Air filters : gives clean bacteria-free air. HEPA filters used in laminar air flow systems. �Syringe filters : syringes fitted with membranes of different diameters.

RADIATIONS � 2 types : Ionizing Non-ionizing

�Ionizing radiations : Gamma rays, X-rays, cosmic rays. High penetrating power, highly lethal. For disposable items like plastic syringes, swabs, culture plates, cannulas, catheters. Cold sterilization. Advantages – speed, high penetrating power, absence of heat.

�Non – ionizing radiations : Infrared, ultraviolet radiations. Infrared – rapid mass sterilization of syringes, catheters. UV rays – bactericidal-denaturation of proteins. disinfects bacteriological laboratories, inoculation hoods, laminar flow, operation theatres.

CHEMICAL METHODS Ideal antiseptic / disinfectant : �Wide spectrum of activity, effective. �Action in presence of organic matters. �High penetration power, quick action. �Stable. Compatibility. �No corrosion of metals, no local irritation, no toxicity. �Safe, easy to use. �Easily available, cheap.

Factors influencing potency of a disinfectant : �Concentration �Time of action �p. H �Temperature �Nature of organism �Presence of organic matter

�Disinfectants are of 3 types : i. High level disinfectants ii. Intermediate level disinfectants iii. Low level disinfectants

High level disinfectants : �Used for endoscopes, cystoscopes, surgical instruments. �Glutaraldehyde, hydrogen peroxide, peracetic acid, chlorine compounds. Intermediate level disinfectants : �Used for instruments like laryngoscopes, endoscopes. �Alcohols, iodophores, phenolic compounds.

Low level disinfectants : �Used for items that come in contact with the patient but do not penetrate into tissue like stethoscopes, electrocardiogram electrodes.

Alcohols �Ethyl alcohol, isopropyl alcohol. �Denature bacterial proteins. �Used as skin antiseptics. �Effective at a concentration of 60 -70%. �Isopropyl alcohol preferred to ethyl alcohol. �Methyl alcohol – fungal spores.

Aldehydes : formaldehyde and glutaraldehyde. Formaldehyde : used in aqueous and gaseous forms. bactericidal, sporicidal, virucidal. �Uses : Preservation of tissue. Sterilization of vaccines. Preparation of toxoid from toxin. Killing bacterial cultures, suspensions. Destroying anthrax spores in hair and wool.

Glutaraldehyde : �Effective against bacteria, fungi, viruses, spores. �Less toxic, less irritant. �Sterilant, high level disinfectant. �Used as 2% buffered solution. �Used for delicate instruments with lenses. �Uses : Cystoscopes, endoscopes, bronchoscope. Plastic endotracheal tubes, face masks, rubber anasthetic tubes, metal instruments.

Phenols �Cell membrane damage, lysis. � 1% phenol – bactericidal. �Absorbed by skin, causes toxicity. �Cresol, chlorhexidine, chloroxylenol, hexachlorophane commonly used.

�Cresols : infected glasswares, cleaning floors, disinfection of excreta. Active against wide range of organisms. �Chlorhexidine : wounds, preoperative disinfection of skin, bladder irrigant. More active against Gram positive bacteria. Good fungicide.

�Chloroxylenol : active ingredient of dettol. Less toxic, less irritant. �Hexachlorophane : active against Gram positive. Prophylaxis against Staphylococcus infections. Potentially toxic.

Halogens �Commonly used – chlorine, iodine. �Bactericidal. �Effective against sporing bacteria, viruses.

Chlorine : �Water supplies, swimming pools, food and dairy industries. �Bleaching powder, sodium hypochlorite, chloramine. �Action – release of free chlorine. �Hypochlorites – bactericidal, fungicidal, virucidal, sporicidal. �Chloramines – antiseptics for dressing wounds.

Iodine : �Skin disinfectant. �Bactericidal, moderate action against spores. �Active against Mycobacterium tuberculosis, viruses. �Iodophores – more active. eg: betadine.

Oxidising Agents �Hydrogen peroxide : effective at conc. 3 -6% �Liberation of free hydroxyl radical on decomposition. �Disinfects contact lenses, surgical prostheses, plastic implants. �Peracetic acid : high level disinfectant. �Also used in plasma sterilization procedure.

�Plasma sterilization : �Plasma – gas with electrons, ions, neutral particles. �H 2 O 2 or H 2 O 2 with peracetic acid – induce plasma. �UV rays – destroy vegetative forms, spores. �Sterrad 100 S steriliser, Plazlyte steriliser. �Sterilize surgical instruments.

Salts �Salts of copper, silver, mercury. �Protein coagulants. �Merthiolate – preservation of sera. �Mercuric chloride – highly toxic. �Thiomersal, mercurochrome – mild antiseptics. �Copper salts – fungicides.

Surface active agents / Surfactants �Anionic, cationic, nonionic, amphoteric. �Cationic – important antibacterial agents. �Loss of membrane semipermeability, protein denaturation. �Quaternary ammonium compounds most important. �Gram positive more susceptible. �Acetyl trimethyl ammonium bromide, benzalkonium chloride.

�Anionic – eg: common soaps. �Strong detergents, weak antimicrobial properties. �Active at acidic p. H. �Effective against Gram positive organisms. �Amphoteric – Tego compounds. �Detergent property, antimicrobial activity. �Active against Gram positive, Gram negative, viruses. �Active over wide range of p. H.

Dyes �Aniline, Acridine dyes – skin, wound antiseptics. �Bacteriostatic. �Aniline dyes – crystal violet, brilliant green, malachite green. �More active against Gram positive bacteria. �Non-toxic, non-irritant. �Interfere with synthesis of peptidoglycan. �Selective agents in culture media.

�Acridine dyes – acriflavine, euflavine, proflavine, aminacrine. �More active against Gram positive. �Used in clinical medicine. �Interfere with synthesis of nucleic acids, proteins. �Gentian violet, acriflavine – skin disinfection.

Vapour Phase Disinfectants �Formaldehyde gas �Fumigation of operation theatres, wards, labs. �Ethylene oxide �Effective against all types of microorganisms. �Sterilises plastic and rubber articles, respirators, heart -lung machines, sutures, dental equipments, clothing. �Used for disposable plastic syringes, petridishes.

�Betapropiolactone �Condensation product of ketane and formaldehyde. �Effective against all microorganisms. �Fumigation. �Inactivation of vaccines.
- Slides: 64