Sterile products and admixtures Dr J Domenech Parenteral



















![Limulus amebocyte lysate [LAL] test • Limulus amebocyte lysate [LAL] test another method for Limulus amebocyte lysate [LAL] test • Limulus amebocyte lysate [LAL] test another method for](https://slidetodoc.com/presentation_image/35287a6ea319c90f6c23c60c7bbdbdb3/image-29.jpg)



- Slides: 32

Sterile products and admixtures Dr. J. Domenech

Parenteral refers administration. injectable route of It derived from Greek words Para (Outside) and enteron (Intestine). So it is a route of administration other than the oral route. This route of administration bypasses the alimentary canal

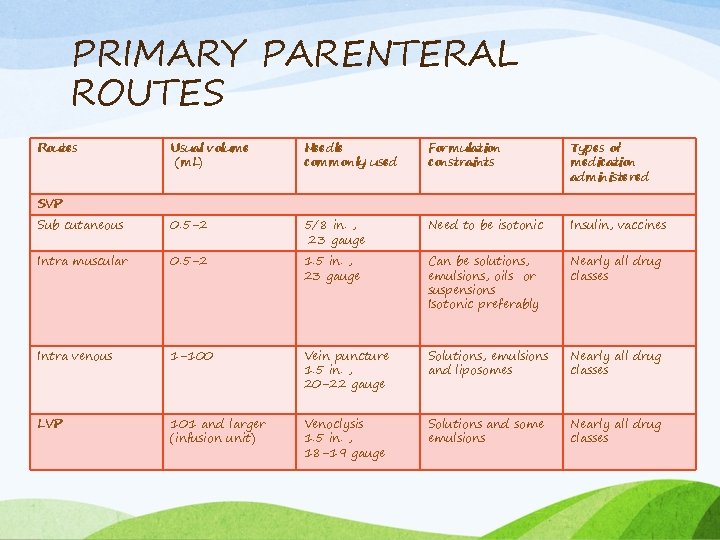
PRIMARY PARENTERAL ROUTES Routes Usual volume (m. L) Needle commonly used Formulation constraints Types of medication administered Sub cutaneous 0. 5 -2 5/8 in. , 23 gauge Need to be isotonic Insulin, vaccines Intra muscular 0. 5 -2 1. 5 in. , 23 gauge Can be solutions, emulsions, oils or suspensions Isotonic preferably Nearly all drug classes Intra venous 1 -100 Vein puncture 1. 5 in. , 20 -22 gauge Solutions, emulsions and liposomes Nearly all drug classes LVP 101 and larger (infusion unit) Venoclysis 1. 5 in. , 18 -19 gauge Solutions and some emulsions Nearly all drug classes SVP

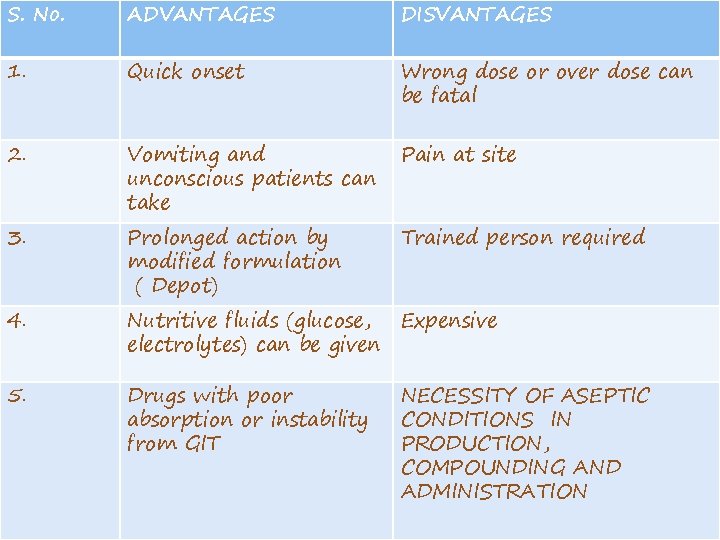
S. No. ADVANTAGES DISVANTAGES 1. Quick onset Wrong dose or over dose can be fatal 2. Vomiting and unconscious patients can take Pain at site 3. Prolonged action by modified formulation ( Depot) Trained person required 4. Nutritive fluids (glucose, Expensive electrolytes) can be given 5. Drugs with poor absorption or instability from GIT NECESSITY OF ASEPTIC CONDITIONS IN PRODUCTION, COMPOUNDING AND ADMINISTRATION

Containers: 1. Glass: • Highly Resistant Borosilicate Glass • Treated Soda lime Glass • Regular Soda Lime Glass • N. P (Non-parenteral) Glass Type 4 is not used for parenteral packaging, others all are used for parenteral packaging.

2. Plastic: Plastic containers are used but they face following problems • Permeation • Sorption • Leaching • Softening 3. Rubber: To provide closure for multiple dose vials, IV fluid bottles, plugs for disposable syringes and bulbs for ophthalmic pipettes, rubber is the material of choice. Problems associated with rubber closures are • Incompatibility • Chemical instability • Physical instability

Closure: • Characteristics of Good Pharmaceutical rubbers • Good ageing qualities • Satisfactory hardness and elasticity • Resistance to sterilization conditions • Impermeable to moisture and air • Examples: • Butyl Rubbers • Natural Rubbers • Neoprene Rubbers • Polyisoprene rubbers • Silicone Rubbers

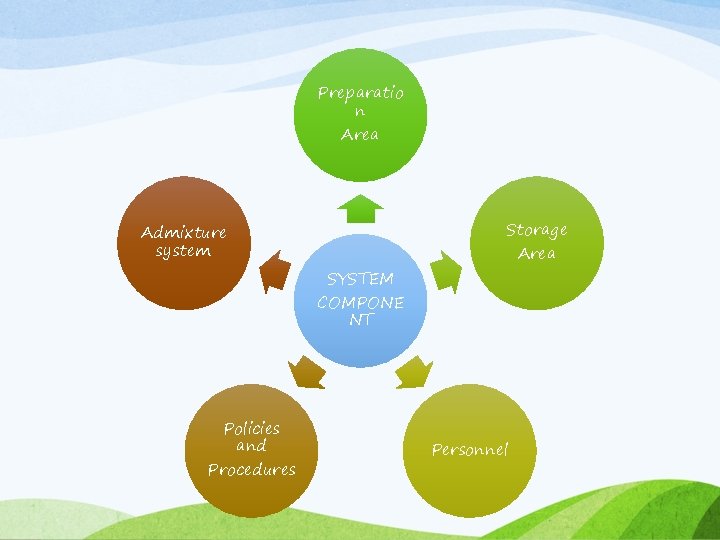
Intravenous Admixture System • “Admixture system” refers to sterile IV solutions that are prepared by using one or more medications or electrolytes and will be administered via the parenteral route. • It requires the measured addition of a medication to a 50 ml or larger bag or bottle of IV fluid. • It can be provided to the patient in his/her home. • Many hospitals involved in compounding IV solutions and medications to outpatient settings.

Methods for safe & effective use of IV admixture • Proper training to nurses & pharmacist • Instruction regarding labeling Information for stability & compatibility to the hospital pharmacy dept. • Information for the formulation skills to the pharmacist.

Preparatio n Area Admixture system SYSTEM COMPONE NT Policies and Procedures Storage Area Personnel

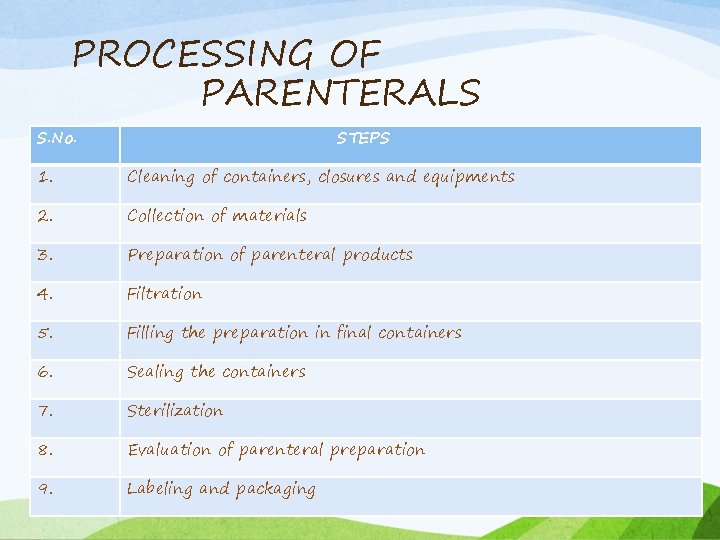
PROCESSING OF PARENTERALS S. No. STEPS 1. Cleaning of containers, closures and equipments 2. Collection of materials 3. Preparation of parenteral products 4. Filtration 5. Filling the preparation in final containers 6. Sealing the containers 7. Sterilization 8. Evaluation of parenteral preparation 9. Labeling and packaging

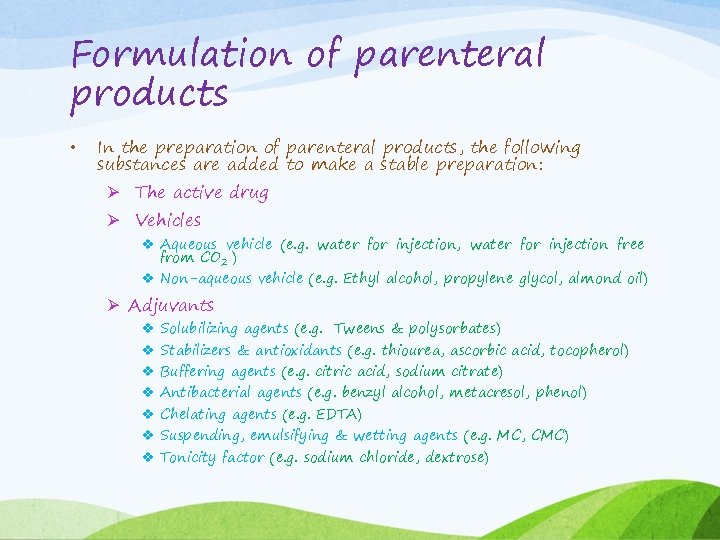
Formulation of parenteral products • In the preparation of parenteral products, the following substances are added to make a stable preparation: Ø The active drug Ø Vehicles v Aqueous vehicle (e. g. water for injection, water for injection free from CO 2 ) v Non-aqueous vehicle (e. g. Ethyl alcohol, propylene glycol, almond oil) Ø Adjuvants v v v v Solubilizing agents (e. g. Tweens & polysorbates) Stabilizers & antioxidants (e. g. thiourea, ascorbic acid, tocopherol) Buffering agents (e. g. citric acid, sodium citrate) Antibacterial agents (e. g. benzyl alcohol, metacresol, phenol) Chelating agents (e. g. EDTA) Suspending, emulsifying & wetting agents (e. g. MC, CMC) Tonicity factor (e. g. sodium chloride, dextrose)

Production facilities of parenterals • The production area where the parenteral preparation are manufactured divided into five sections: v Clean-up area v Preparation area v Aseptic area v Quarantine area v Finishing & packaging area can be

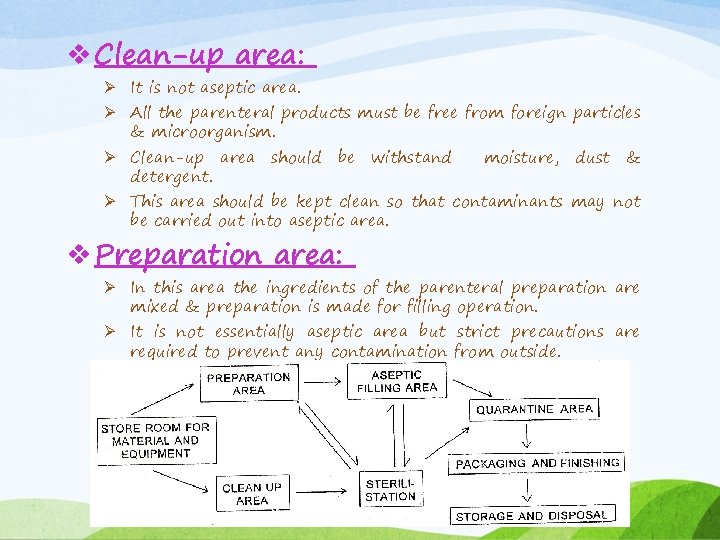
v Clean-up area: Ø It is not aseptic area. Ø All the parenteral products must be free from foreign particles & microorganism. Ø Clean-up area should be withstand detergent. moisture, dust & Ø This area should be kept clean so that contaminants may not be carried out into aseptic area. v Preparation area: Ø In this area the ingredients of the parenteral preparation are mixed & preparation is made for filling operation. Ø It is not essentially aseptic area but strict precautions are required to prevent any contamination from outside.

v Aseptic area: Ø The parenteral preparations are filtered, filled into final container & sealed should be in aseptic area. Ø The entry of personnel into aseptic area should be limited & through an air lock. Ø Ceiling, wall & floor of that area should be sealed & painted. Ø The air in the aseptic area should be free from fibers, dust and microorganism. Ø The High efficiency particulate air filters (HEPA) is used for air. Ø UV lamps are fitted in order to maintain sterility.

v. Quarantine area: v After filling, sealing & sterilization the parenteral product are held up in quarantine area. v Randomly samples were kept foe evaluation. v The batch or product pass the evaluation tests are transfer in to finishing or packaging area. v Finishing & packaging area: v Parenteral products are properly labelled and packed. v Properly packing is essential to provide protection against physical damage. v The labelled container should be packed in cardboard or plastic container. v Ampoules should be packed in partitioned boxes

EVALUATION OF PARENTERAL PREPARATIONS • The finished parenteral products are subjected to the following tests, in order to maintain quality control: • A) sterility test • B)clarity test • C)leakage test • D)pyrogen test • E)assay

A) sterility test • It is a procedure carried out to detect and conform absence of any viable form of microbes in or on pharmacopeia preparation or product. 1) Method of sterility testing i ) METHOD 1 Membrane filtration method ii) METHOD 2 Direct inoculation method

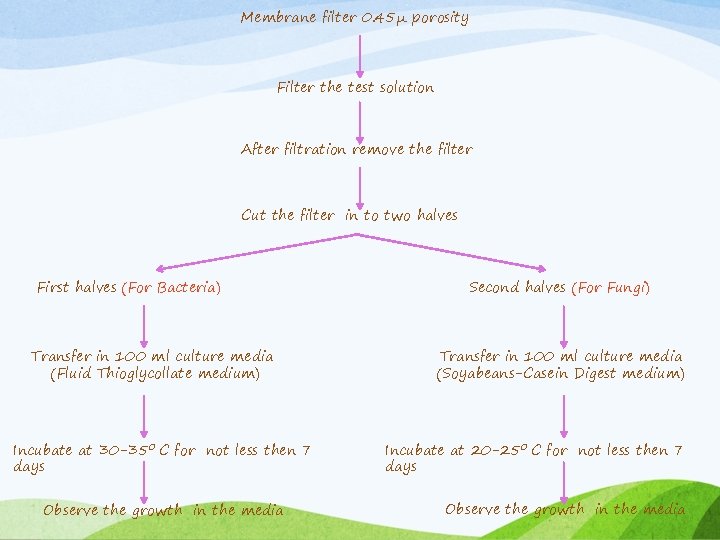
Membrane filtration method (METHOD 1): v Membrane filtration Appropriate for : (advantage) • Filterable aqueous preparations • Alcoholic preparations • Oily preparations • Preparations miscible with or soluble in aqueous or oily (solvents with no antimicrobial effect) v All steps of this procedure are performed aseptically in a Class 100 Laminar Flow Hood

Membrane filter 0. 45μ porosity Filter the test solution After filtration remove the filter Cut the filter in to two halves First halves (For Bacteria) Transfer in 100 ml culture media (Fluid Thioglycollate medium) Incubate at 30 -350 C for not less then 7 days Observe the growth in the media Second halves (For Fungi) Transfer in 100 ml culture media (Soyabeans-Casein Digest medium) Incubate at 20 -250 C for not less then 7 days Observe the growth in the media

Direct inoculation method (METHOD 2): v Suitable for samples with small volumes v volume of the product is not more than 10% of the volume of the medium v suitable method for aqueous solutions, oily liquids, ointments and creams v Direct inoculation of the culture medium suitable quantity of the preparation to be examined is transferred directly into the appropriate culture medium & incubate for not less than 14 days.

Observation and results Culture media is examined during and after at the end of incubation. The following observations are possible: 1) No evidence of growth 2) There is evidence of growth Re-testing is performed same no. of sample, volume & media as in original test of growth 3) Pass the test for sterility. There is evidence of growth No evidence isolate & identify the organism. Re-testing is performed with twice no. of sample if: No evidence of growth Pass the test for sterility.

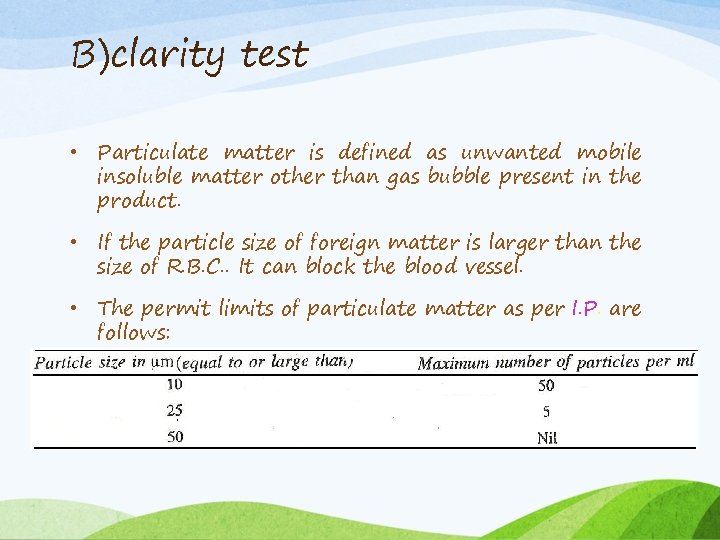
B)clarity test • Particulate matter is defined as unwanted mobile insoluble matter other than gas bubble present in the product. • If the particle size of foreign matter is larger than the size of R. B. C. . It can block the blood vessel. • The permit limits of particulate matter as per I. P. are follows:

Methods for monitoring particulate matter contamination: 1) Visual method 2) Coulter counter method 3) Filtration method 4) Light blockage method

C)leakage test • The sealed ampoules are subjected to small cracks which occur due to rapid temperature changes or due to mechanical shocks. Filled & sealed ampoules Dipped in 1% Methylene blue solution Under negative pressure in vacuum chamber Vacuum released colored solution enter into the ampoule Defective sealing Vials & bottles are not suitable for this test because the sealing material used is not rigid

D)pyrogen test v Pyrogen = “Pyro” (Greek = Fire) + “gen” (Greek = beginning). v Fever producing, metabolic microbial growth and death. by-products of v Bacterial pyrogens are called “Endotoxins”. Gram negative bacteria produce more potent endotoxins than gram + bacteria and fungi. v Endotoxins are heat stable lipopolysaccharides (LPS) present in bacterial cell walls, not present in cell-free bacterial filtrates

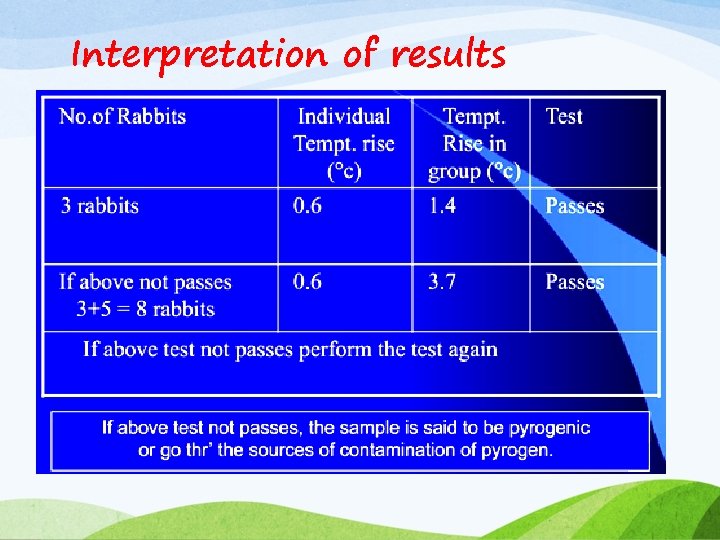
Method v Dissolve the subs being examined in, or dilute it with a pyrogen free saline solution v Warm the liquid being examined to approx. 38. 5 o C temp before injection v The volume of injection is NLT 0. 5 ml/kg & NMT 10 ml/kg of body weight v Withhold water during test v Clinical thermometer is inserted into the rectum of rabbit to record body temp v 2 normal reading of rectal temp are should be taken prior to the test injection at an interval of half an hr & its mean is calculated- initial temp v The solution under test is injected through an ear vein v Record the temp of each rabbit in an interval of 30 min for 3 hrs v The difference between initial temp & maximum temp is recorded- taken as response

Interpretation of results
![Limulus amebocyte lysate LAL test Limulus amebocyte lysate LAL test another method for Limulus amebocyte lysate [LAL] test • Limulus amebocyte lysate [LAL] test another method for](https://slidetodoc.com/presentation_image/35287a6ea319c90f6c23c60c7bbdbdb3/image-29.jpg)
Limulus amebocyte lysate [LAL] test • Limulus amebocyte lysate [LAL] test another method for the determination of pyrogenic endotoxins • In this method the test solution is combined with a cell lysate from the ameabocyte [blood celels] of horse shoe crab • Any endo toxin that might be present will be coagulated with protien fraction of the ameabocytes and results in the formation of a gel • This consider to be simple, rapid and of greater sensitivity that the rabbit test

E)assay • Assay is performed according to method given In the monograph of that parental preperation in the pharmacopoeia • Assay is done to check the quantity of medicament present in the parenteral preperation

References • Encyclopedia of pharmaceutical technology by James Swarbrick pg. no. 1266 -1299 • Pharmaceutical product development by N. K. JAIN • Chemical Incompatibility of Parenteral Drug Admixtures; T. J. Mccarthy; S. A. Medical journal 2 • The theory & pratice of “Industrial Pharmacy” Leon Lachman , Herbert A. Liberman. special Indian Edition 2009 Pg. No. 693 -680. • Modern Pharmaceutics Fourth Edition, Revised and Expanded, Edited By G. S. Banker & C. T. Rhodes, Marcel Dekker pg 387 -389. • The Science & practice of Pharmacy, By Remington, Vol 01, Edi. 21 st, Lippincott Publication, pg-838 -840.

Thank You……. .
 Iv admixture preparation
Iv admixture preparation Sterile vs non sterile compounding
Sterile vs non sterile compounding Sterile products and aseptic techniques
Sterile products and aseptic techniques Sterile products and aseptic techniques
Sterile products and aseptic techniques Sterile products and aseptic techniques
Sterile products and aseptic techniques Gmp sterile pharmaceutical products
Gmp sterile pharmaceutical products Gmp for sterile pharmaceutical products
Gmp for sterile pharmaceutical products Dal i
Dal i Institut canet de mar
Institut canet de mar Santeisation
Santeisation Joseph domenech
Joseph domenech Donde nacio salvador dali
Donde nacio salvador dali Pipeed
Pipeed Concrete admixtures
Concrete admixtures Concrete admixtures
Concrete admixtures Astm c 1644
Astm c 1644 Air entrainment
Air entrainment Hrwr concrete admixture
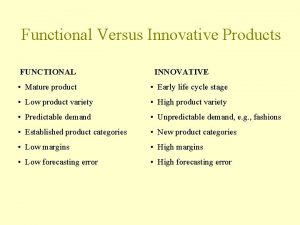
Hrwr concrete admixture Functional products
Functional products Coke vs pepsi products
Coke vs pepsi products Usp 797 laminar flow hood cleaning
Usp 797 laminar flow hood cleaning Instrument processing optimization
Instrument processing optimization Sterile pyuria
Sterile pyuria Sterile gloves
Sterile gloves Podning af lupiner
Podning af lupiner Sterile technique quiz
Sterile technique quiz Sterile compounding calculations
Sterile compounding calculations Chapter 15:4 observing standard precautions
Chapter 15:4 observing standard precautions Sterile flower
Sterile flower What process is this
What process is this Comminution compounding
Comminution compounding Chapter 15:3 washing hands
Chapter 15:3 washing hands Chapter 15:8 using sterile techniques
Chapter 15:8 using sterile techniques