Sterile Body Fluids Normally sterile body fluids Rapid


Sterile Body Fluids
Normally sterile body fluids • Rapid and accurate microbiological assessment of normally sterile body fluids samples is important for successful patient management. • Any microorganism that is not microbiota should be considered significant and some flora that may inter.

Sterile Body Fluids Ø CSF Ø Serous (pleural, peritoneal, pericardial) Ø Synovial fluids

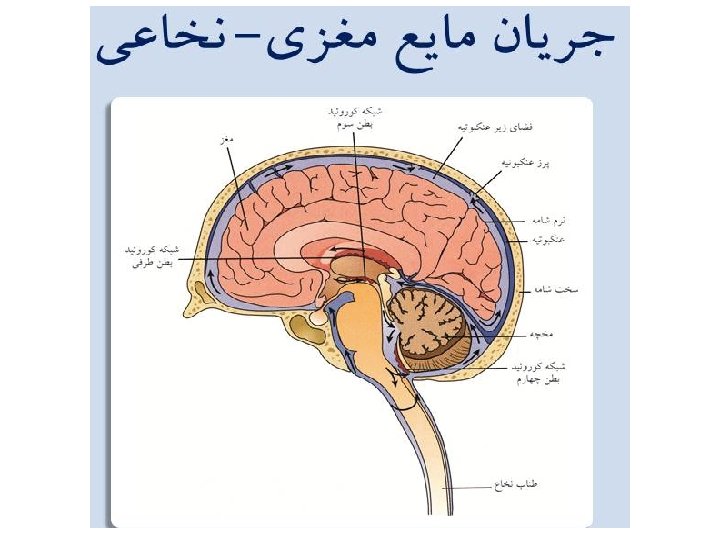
Cerebrospinal fluid (CSF)

CSF • Central Nervous System (CNS): • Brain & Spinal cord • Diagnosis of an infection involving the CNS is of critical importance. • Most clinicians consider infection in the CNS to be a medical emergency.


Routes of Infection • One of the most important defense mechanisms of the CNS is the blood-brain barrier. • Organisms may gain access to the CNS through several primary routes: • Hematogenous spread: the most common route of infection for the CNS. • Direct spread from an infected site: The extension of an infection close to or contiguous with the CNS: otitis media and sinusitis • Anatomic defects in CNS structures: surgery, trauma, or congenital abnormalities • Travel along nerves leading to the brain: occurs with organisms such as rabies virus and herpes simplex virus.
Meningitis • Based on the host’s response to the invading microorganism, meningitis is divided into two major categories: purulent and aseptic meningitis. • Purulent Meningitis: CSF containing large numbers of polymorphonuclear cells (PMNs) and bacterial are usually cause of infection. - newborns: group B streptococci, E. coli, and L. monocytogenes, H. influenzae - adults: N. meningitidis, S. pneumoniae • Aseptic Meningitis: usually viral and is characterized by an increase of lymphocytes and other mononuclear cells in the CSF


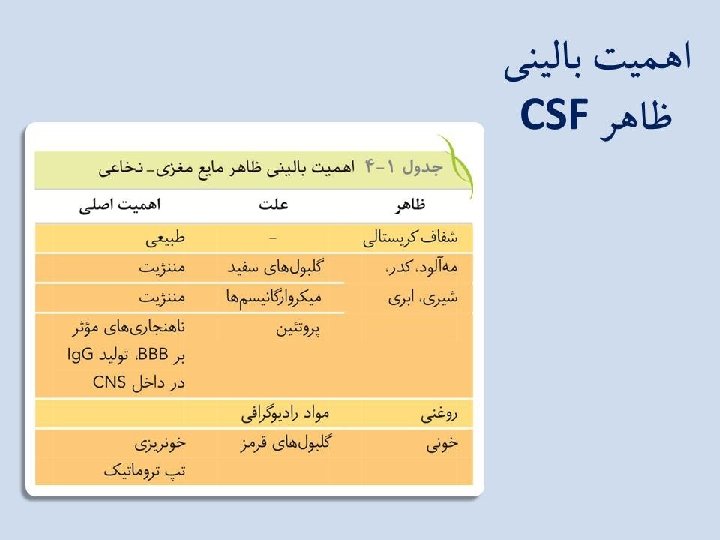
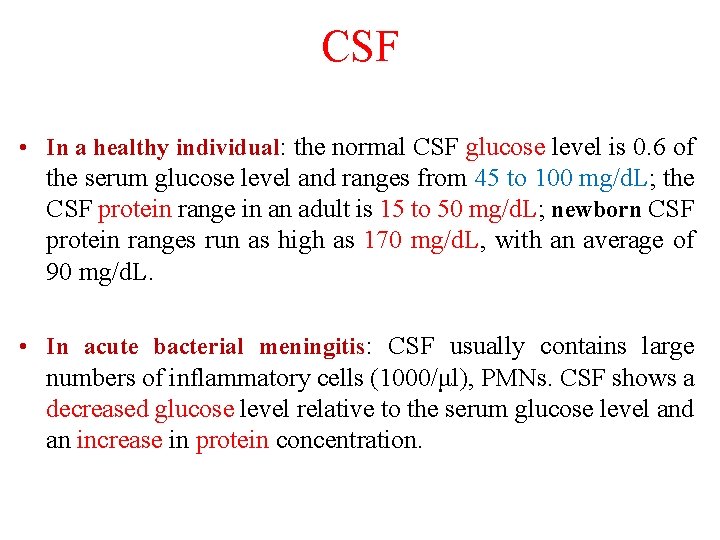
CSF • In a healthy individual: the normal CSF glucose level is 0. 6 of the serum glucose level and ranges from 45 to 100 mg/d. L; the CSF protein range in an adult is 15 to 50 mg/d. L; newborn CSF protein ranges run as high as 170 mg/d. L, with an average of 90 mg/d. L. • In acute bacterial meningitis: CSF usually contains large numbers of inflammatory cells (1000/μl), PMNs. CSF shows a decreased glucose level relative to the serum glucose level and an increase in protein concentration.
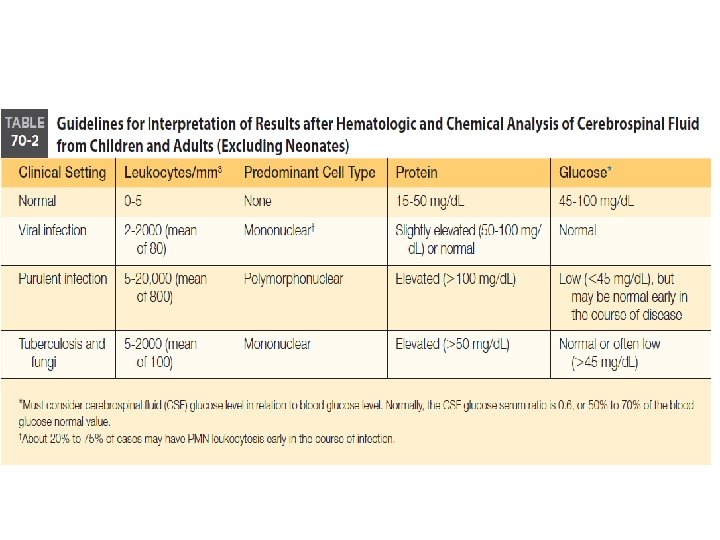

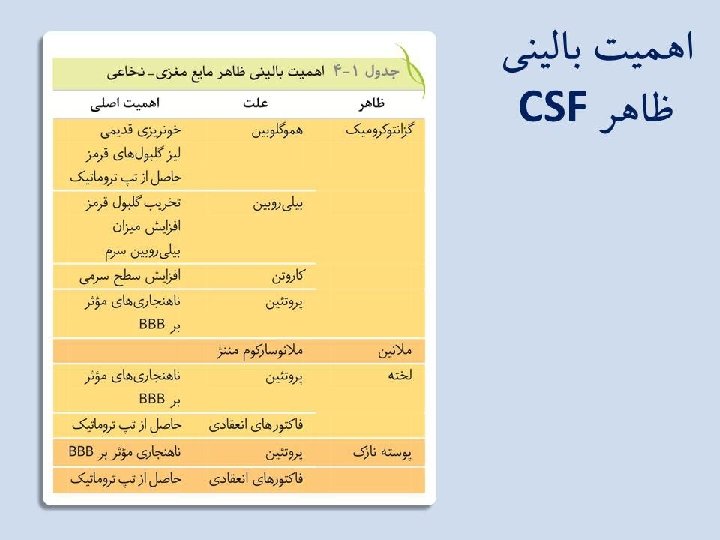
Differential Diagnosis of Meningitis by Laboratory Results Bacterial Viral Tubercular Fungal Increased WBC count Neutrophils Lymphs Lymps & Monos Lymphs & Monos Marked ↑ protein Mod-Marked ↑ protein Marked ↓ glucose ↔ normal glucose ↓ glucose Normal to ↓ glucose Lactate > 35 mg/d. L Lactate normal 11 -22 mg/d. L Lactate > 25 mg/d. L + gram stains + India ink with Cryptococcus neoformans + bacterial antigen tests + immunological test for C. neo.
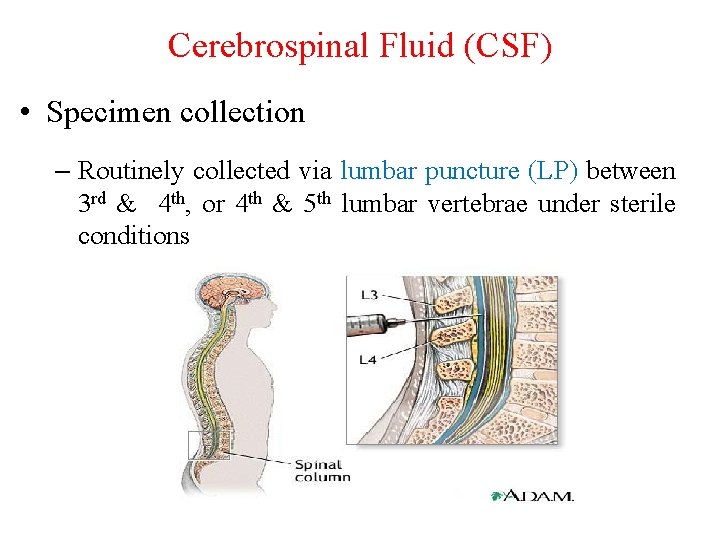
Cerebrospinal Fluid (CSF) • Specimen collection – Routinely collected via lumbar puncture (LP) between 3 rd & 4 th, or 4 th & 5 th lumbar vertebrae under sterile conditions

Cerebrospinal Fluid (CSF) • Specimen collection • Tube 1 – chemistries and serology • Tube 2 – microbiology cultures • Tube 3 – hematology • 1 -3 m. L in each tube A minimum of 5 to 10 m. L is recommended for detecting mycobacteria and fungi.

CSF • CSF should be hand-delivered immediately to the laboratory. • Certain agents, such as S. pneumoniae, may not be detectable after an hour or longer. • Specimens for microbiology studies should never be refrigerated; if not rapidly processed, CSF should be incubated (35°C) or left at room temperature. • One exception is for viral studies. Specimens may be refrigerated for as long as 24 hours after collection or frozen at -70°C in longer delay • If not processed immediately, CSF specimen for hematology studies can be refrigerated, whereas the CSF for chemistry and serology can be frozen (-20°C).
CSF • Initial processing of CSF for bacterial, fungal, or parasitic studies includes centrifugation of all specimens with a volume greater than 1 m. L for at least 15 minutes at 1500 g. • If less than 1 m. L of CSF is available, the specimens should be Gram stained and plated directly to blood and chocolate agar plates. The supernatant is removed to a sterile tube, leaving approximately 0. 5 m. L of fluid for visual examination or culture. • Supernatant can be used to test for the presence of antigens or for chemistry evaluations
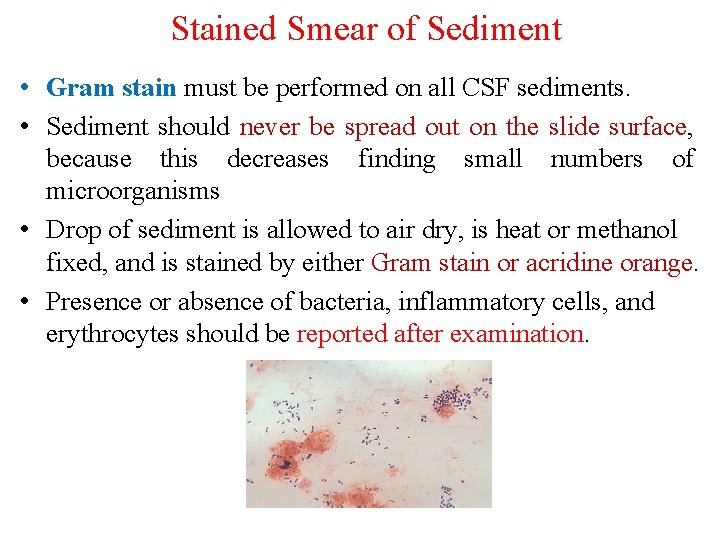
Stained Smear of Sediment • Gram stain must be performed on all CSF sediments. • Sediment should never be spread out on the slide surface, because this decreases finding small numbers of microorganisms • Drop of sediment is allowed to air dry, is heat or methanol fixed, and is stained by either Gram stain or acridine orange. • Presence or absence of bacteria, inflammatory cells, and erythrocytes should be reported after examination.

Wet Preparation • Amoebas are best observed by examining thoroughly mixed sediment as a wet preparation under phasecontrast microscopy. • If a phase-contrast microscope is not available, observing under light microscopy with the condenser close slightly can be used as an alternative.
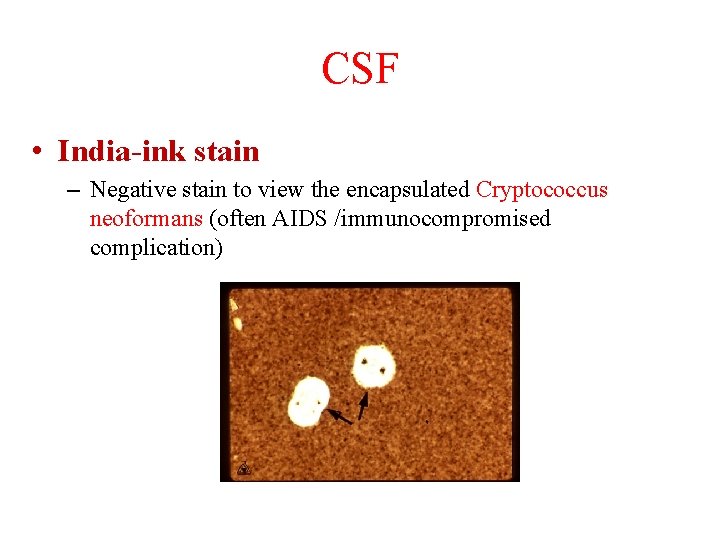
CSF • India-ink stain – Negative stain to view the encapsulated Cryptococcus neoformans (often AIDS /immunocompromised complication)
Direct Detection of Etiologic Agents - Antigen: • Commercial reagents and kits are available for the rapid detection of antigen in the CSF • Rapid antigen detection from CSF has been largely accomplished by the techniques of latex agglutination • All commercial agglutination systems use the principle of an antibody-coated particle capable of binding to specific antigen, resulting in macroscopically visible agglutination. - Molecular Methods: • PCR testing for HSV, EBV, CMV, and enterovirus in CNS infections has a sensitivity nearing 100%
Culture • Bacteriologic media should include a chocolate agar plate, 5% sheep blood agar plate, and an enrichment broth, usually thioglycolate. • Chocolate agar plate is needed to recover fastidious organisms H. influenzae and N. meningitidis, which are unable to grow on blood agar plates • After vortexing sediment and preparing smears, several drops of the sediment should be inoculated to each medium. • Plates should be incubated at 37°C in 5% to 10% CO 2 for at least 72 hours. • If a CO 2 incubator is not available, use a candle jar. • Broth should be incubated in air at 37°C for at least 5 to 10 days. Broth cap must be loose to allow free exchange of air. • If a brain abscess is suspected, an anaerobic blood agar plate may also be inoculated.

Culture • For CSF fungal cultures, two drops of well-mixed sediment should be inoculated on to Sabouraud dextrose agar or brain-heart infusion with 5% sheep blood. • Fungal media should be incubated in at 28 -30°C for 4 weeks.

Serous fluids
Serous fluids • Pleural fluid • Pericardial fluid • Peritonial fluid – acts as lubricant – provide nutrients – remove wastes

Production Re-absorption
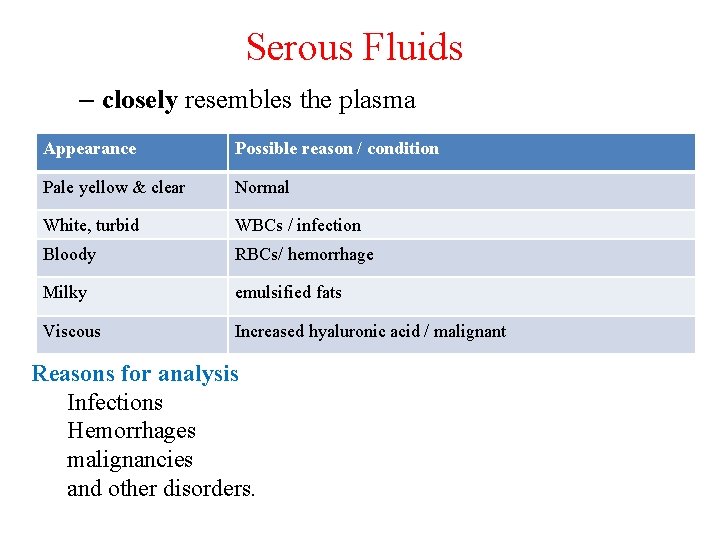
Serous Fluids – closely resembles the plasma Appearance Possible reason / condition Pale yellow & clear Normal White, turbid WBCs / infection Bloody RBCs/ hemorrhage Milky emulsified fats Viscous Increased hyaluronic acid / malignant Reasons for analysis Infections Hemorrhages malignancies and other disorders.

Serous Fluids • Pleural fluid: It is normally about 1 -10 ml • pericardial fluid: In a healthy individual there is usually 15 -50 ml of clear fluid • Peritonial fluid: Up to 50 ml fluid normally present in peritoneal cavity

Serous Fluids • Effusion – an increase in the serous fluid due to some disruption in production &/ re-absorption processes. – Classification of cause of an effusion is aided by determining if the fluid is a “transudate” or an “exudate”.

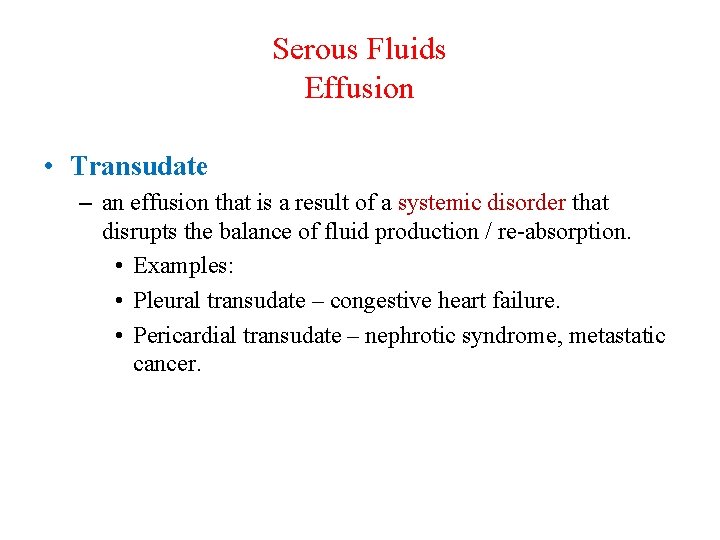
Serous Fluids Effusion • Transudate – an effusion that is a result of a systemic disorder that disrupts the balance of fluid production / re-absorption. • Examples: • Pleural transudate – congestive heart failure. • Pericardial transudate – nephrotic syndrome, metastatic cancer.
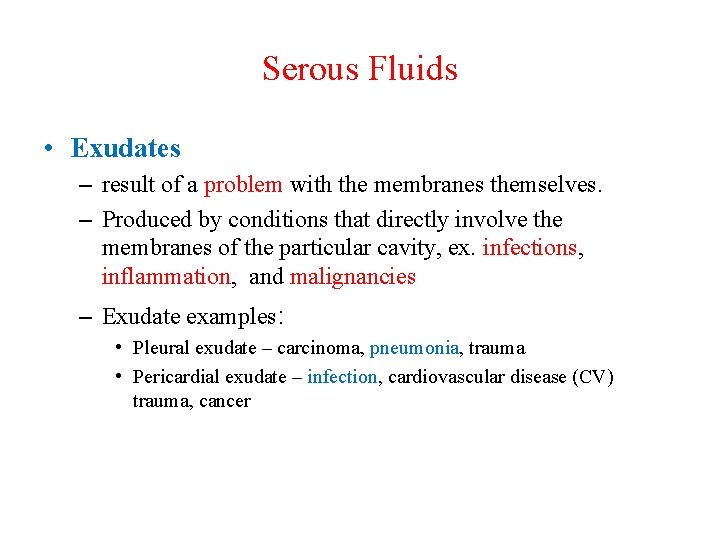
Serous Fluids • Exudates – result of a problem with the membranes themselves. – Produced by conditions that directly involve the membranes of the particular cavity, ex. infections, inflammation, and malignancies – Exudate examples: • Pleural exudate – carcinoma, pneumonia, trauma • Pericardial exudate – infection, cardiovascular disease (CV) trauma, cancer
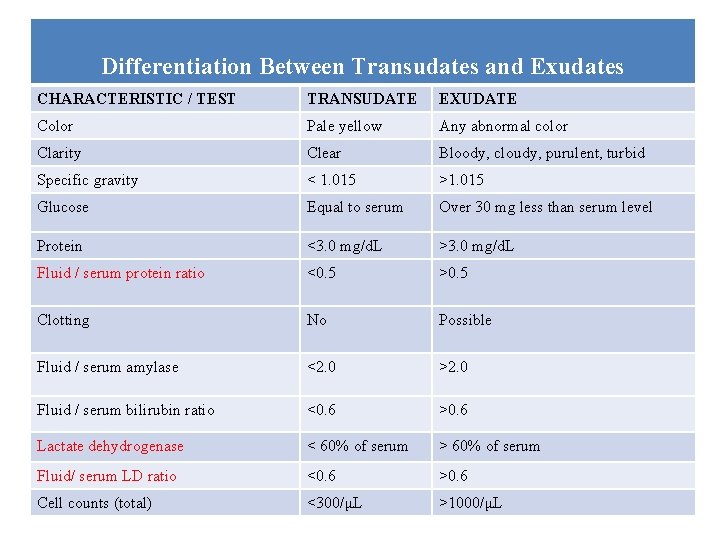
Differentiation Between Transudates and Exudates CHARACTERISTIC / TEST TRANSUDATE EXUDATE Color Pale yellow Any abnormal color Clarity Clear Bloody, cloudy, purulent, turbid Specific gravity < 1. 015 >1. 015 Glucose Equal to serum Over 30 mg less than serum level Protein <3. 0 mg/d. L >3. 0 mg/d. L Fluid / serum protein ratio <0. 5 >0. 5 Clotting No Possible Fluid / serum amylase <2. 0 >2. 0 Fluid / serum bilirubin ratio <0. 6 >0. 6 Lactate dehydrogenase < 60% of serum > 60% of serum Fluid/ serum LD ratio <0. 6 >0. 6 Cell counts (total) <300/μL >1000/μL
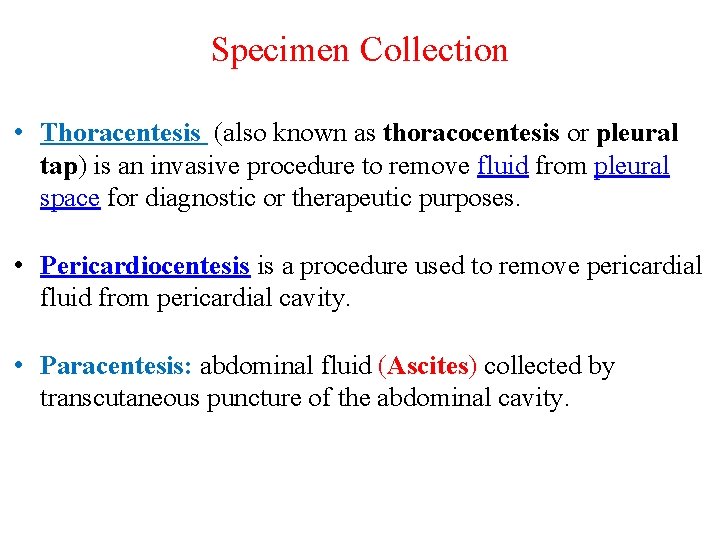
Specimen Collection • Thoracentesis (also known as thoracocentesis or pleural tap) is an invasive procedure to remove fluid from pleural space for diagnostic or therapeutic purposes. • Pericardiocentesis is a procedure used to remove pericardial fluid from pericardial cavity. • Paracentesis: abdominal fluid (Ascites) collected by transcutaneous puncture of the abdominal cavity.
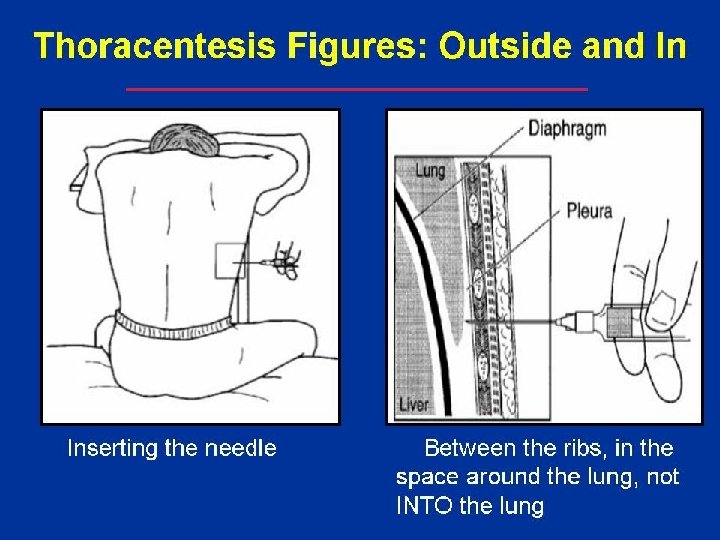
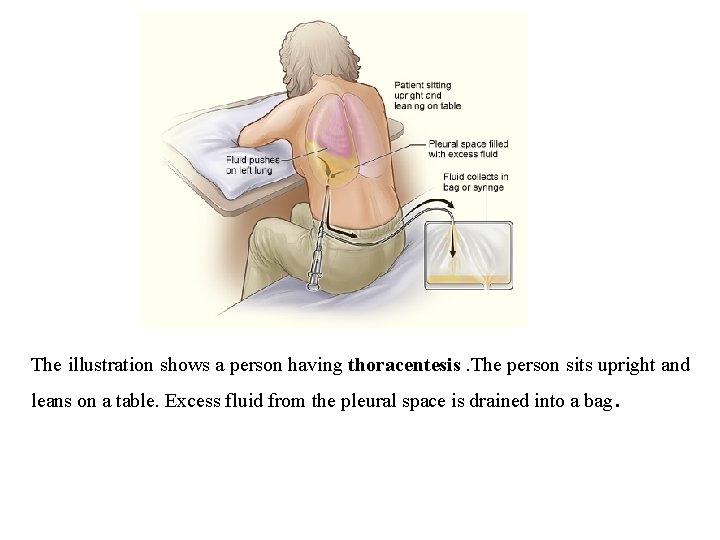
The illustration shows a person having thoracentesis. The person sits upright and leans on a table. Excess fluid from the pleural space is drained into a bag .
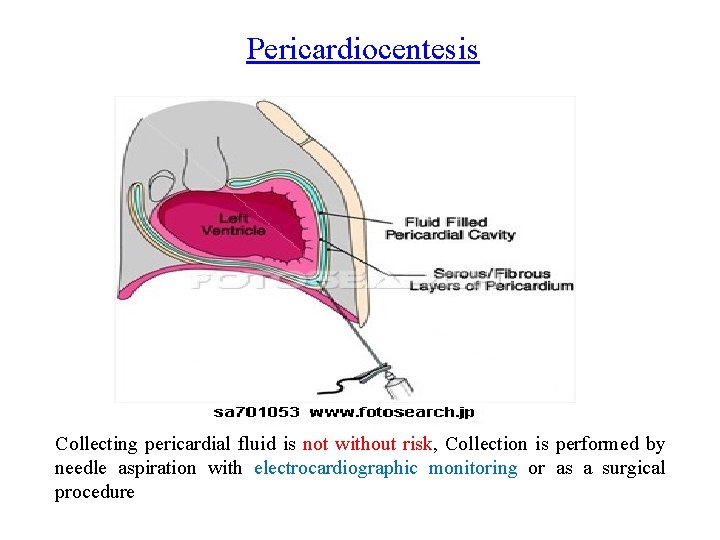
Pericardiocentesis Collecting pericardial fluid is not without risk, Collection is performed by needle aspiration with electrocardiographic monitoring or as a surgical procedure
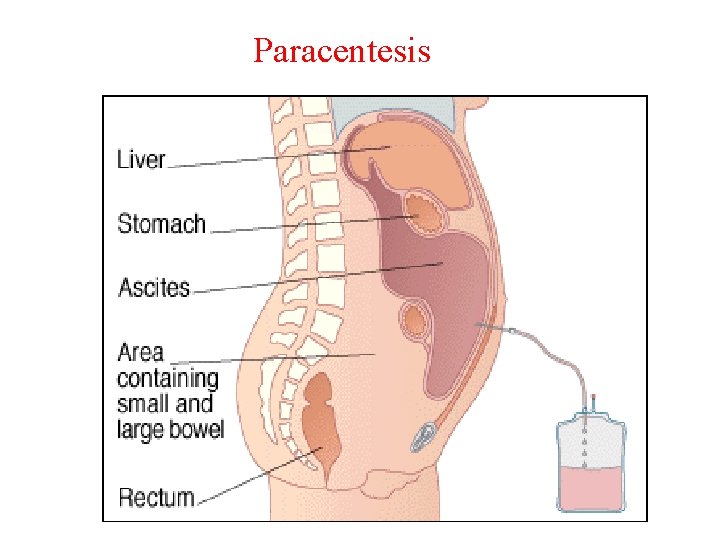
Paracentesis
Serous Fluids • Specimen Collection - EDTA tube for cell count & differential – Heparin tube for chemistries, serology, microbiology and cytology. – Since procedure not performed unless an effusion exists, large amount of fluid often collected. – Blood specimens usually collected at same time and comparisons of test results made.
Serous Fluids - Testing overview – Variety of tests used to aid in determining the cause of the effusion • • Appearance Evaluation of clotting ability whether or not it will form a clot, etc. Cell counts Protein level – Both fluid and current serum level to make comparison: fluid protein / serum protein • LDH enzymes – Both fluid and current serum level to make comparison: fluid LDH/ serum LDH • Cultures • Serology – rarely done on serous fluids as blood testing is adequate • Cytology / Pathology – if malignancy is suspected.

Synovial Fluid
Synovial Fluid • Composition and formation – Secreted by cells of synovial membrane – Very viscous, clear ultrafiltrate of plasma – Contains • • Hyaluronic acid Mucopolysaccharides Limited amount of plasma protein Glucose & uric acid levels equivalent to plasma - Normal amount of fluid contained in the knee cavity is less than 3. 5 ml, this amount increases in disorders of the joints (up to 25 ml).
Synovial Fluid • Functions – supplies nutrients – lubrication of joint • Reasons for analysis – Infection – Hemorrhage – Degenerative disorders (arthritis) – Inflammatory disease
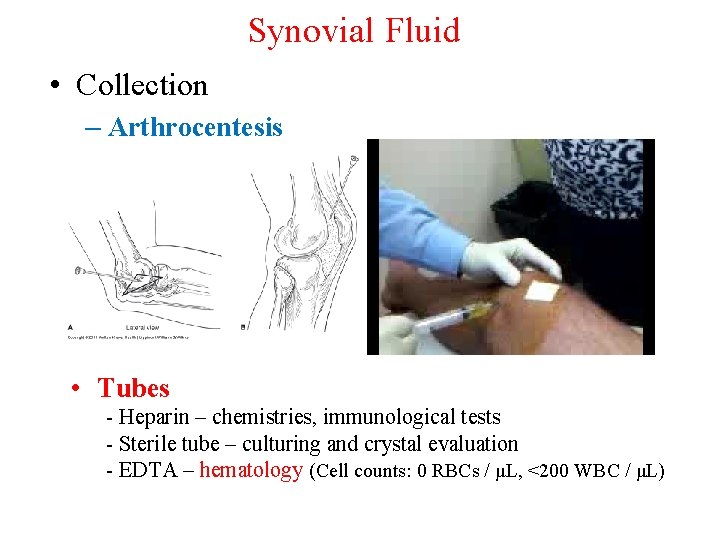
Synovial Fluid • Collection – Arthrocentesis • Tubes - Heparin – chemistries, immunological tests - Sterile tube – culturing and crystal evaluation - EDTA – hematology (Cell counts: 0 RBCs / μL, <200 WBC / μL)
Classification of Synovial Fluid – Normal – Non-Inflammatory • Degenerative joint diseases – Inflammatory • Immunologic disorders ( ie lupus, RA, gout crystals) – Septic • Microbial infections – Hemorrhagic • Traumatic injury, tumors, hemophilia.
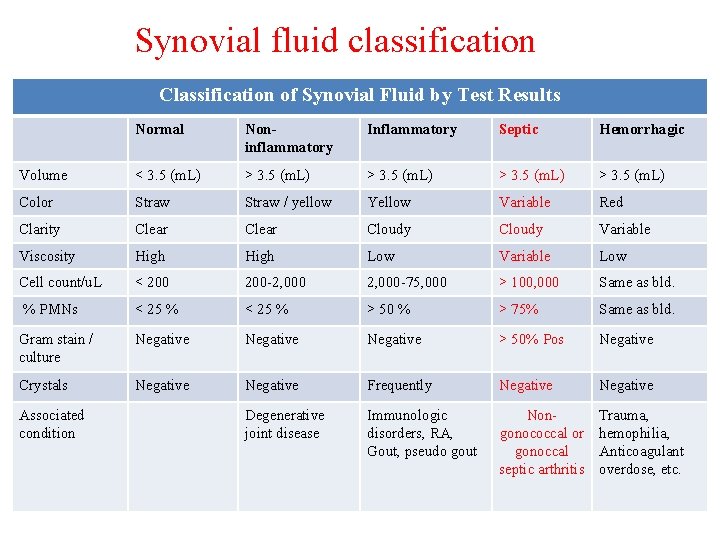
Synovial fluid classification Classification of Synovial Fluid by Test Results Normal Noninflammatory Inflammatory Septic Hemorrhagic Volume < 3. 5 (m. L) > 3. 5 (m. L) Color Straw / yellow Yellow Variable Red Clarity Clear Cloudy Variable Viscosity High Low Variable Low Cell count/u. L < 200 -2, 000 -75, 000 > 100, 000 Same as bld. % PMNs < 25 % > 50 % > 75% Same as bld. Gram stain / culture Negative > 50% Pos Negative Crystals Negative Frequently Negative Degenerative joint disease Immunologic disorders, RA, Gout, pseudo gout Nongonococcal or gonoccal septic arthritis Trauma, hemophilia, Anticoagulant overdose, etc. Associated condition

Pleural, peritoneal, pericardial and synovial fluids culture
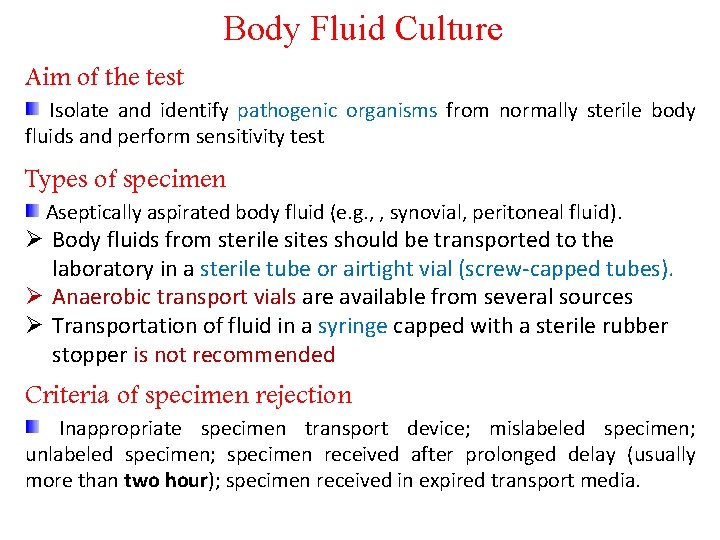
Body Fluid Culture Aim of the test Isolate and identify pathogenic organisms from normally sterile body fluids and perform sensitivity test Types of specimen Aseptically aspirated body fluid (e. g. , , synovial, peritoneal fluid). Ø Body fluids from sterile sites should be transported to the laboratory in a sterile tube or airtight vial (screw-capped tubes). Ø Anaerobic transport vials are available from several sources Ø Transportation of fluid in a syringe capped with a sterile rubber stopper is not recommended Criteria of specimen rejection Inappropriate specimen transport device; mislabeled specimen; unlabeled specimen; specimen received after prolonged delay (usually more than two hour); specimen received in expired transport media.
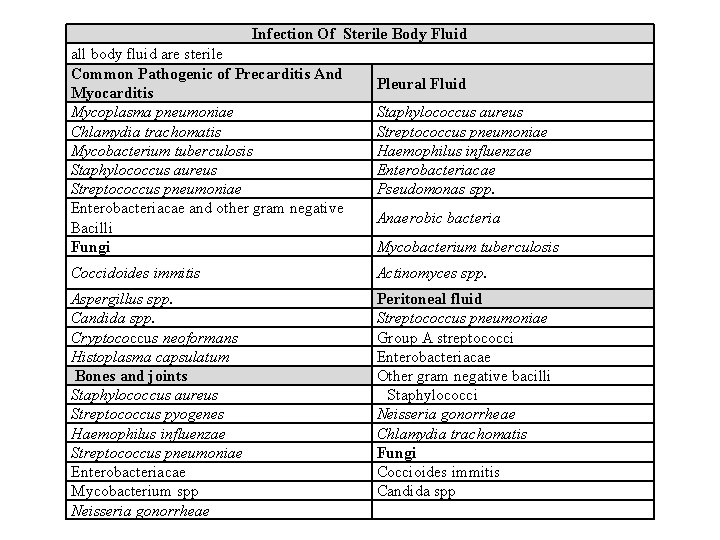
Infection Of Sterile Body Fluid all body fluid are sterile Common Pathogenic of Precarditis And Myocarditis Mycoplasma pneumoniae Chlamydia trachomatis Mycobacterium tuberculosis Staphylococcus aureus Streptococcus pneumoniae Enterobacteriacae and other gram negative Bacilli Fungi Pleural Fluid Staphylococcus aureus Streptococcus pneumoniae Haemophilus influenzae Enterobacteriacae Pseudomonas spp. Anaerobic bacteria Mycobacterium tuberculosis Coccidoides immitis Actinomyces spp. Aspergillus spp. Candida spp. Cryptococcus neoformans Histoplasma capsulatum Bones and joints Staphylococcus aureus Streptococcus pyogenes Haemophilus influenzae Streptococcus pneumoniae Enterobacteriacae Mycobacterium spp Neisseria gonorrheae Peritoneal fluid Streptococcus pneumoniae Group A streptococci Enterobacteriacae Other gram negative bacilli Staphylococci Neisseria gonorrheae Chlamydia trachomatis Fungi Coccioides immitis Candida spp
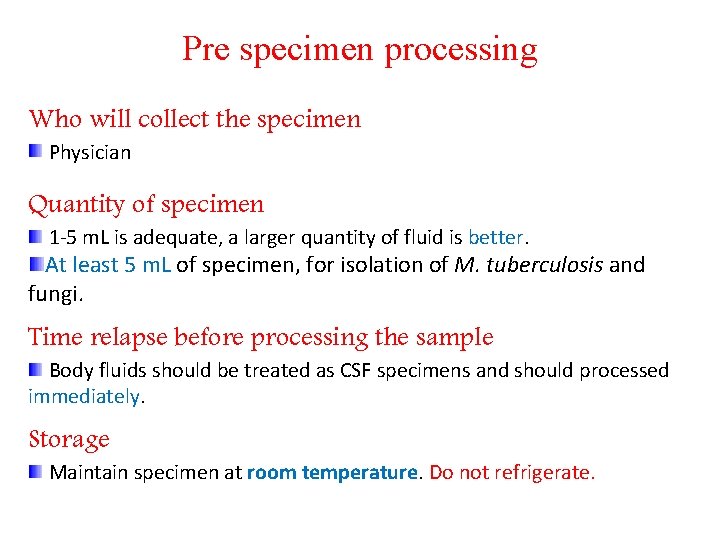
Pre specimen processing Who will collect the specimen Physician Quantity of specimen 1 -5 m. L is adequate, a larger quantity of fluid is better. At least 5 m. L of specimen, for isolation of M. tuberculosis and fungi. Time relapse before processing the sample Body fluids should be treated as CSF specimens and should processed immediately. Storage Maintain specimen at room temperature. Do not refrigerate.
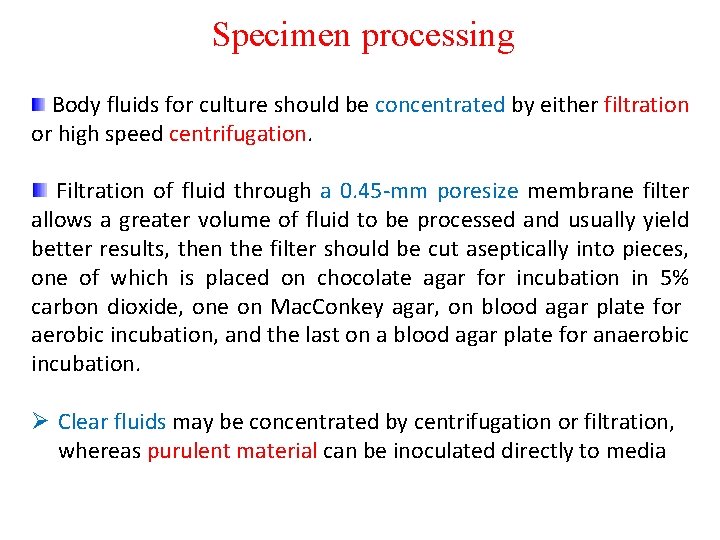
Specimen processing Body fluids for culture should be concentrated by either filtration or high speed centrifugation. Filtration of fluid through a 0. 45 -mm poresize membrane filter allows a greater volume of fluid to be processed and usually yield better results, then the filter should be cut aseptically into pieces, one of which is placed on chocolate agar for incubation in 5% carbon dioxide, one on Mac. Conkey agar, on blood agar plate for aerobic incubation, and the last on a blood agar plate for anaerobic incubation. Ø Clear fluids may be concentrated by centrifugation or filtration, whereas purulent material can be inoculated directly to media
Specimen processing If fluid has been concentrated by centrifugation, the resulting sediment should be inoculated to an enrichment broth, blood, chocolate and Mac. Conkey agars. All fluids should be processed for direct microscopic examination, in general if one organism is seen per oil immersion field at least 105 organisms per milliliter of specimen are present. Specimens for fungi should be examined by direct wet preparation or by preparing 10% KOH for visualization of fungi element from a wet preparation. Acid Fast stain for Mycobacterium spp.
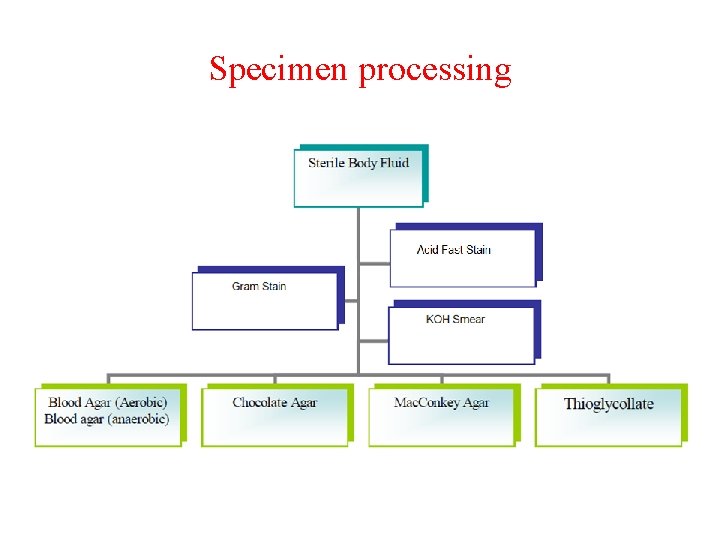
Specimen processing

Culture Ø Inoculate on agar media and streak culture Ø Inoculate in broth media Ø Incubate Blood agar, Mac. Conkey agar, and THIO at 35 -37 °C in an ambient air. Ø Incubate Chocolate agar at 35 -37 °C in a candle jar or CO 2 incubator. Ø Incubate anaerobic plates at 35 -37 °C in an anaerobe jar with gas pack.

Culture 1) Examine all aerobic media after overnight incubation. - If there is no growth on the plates and the THIO broth and BHI is clear, re-incubate and examine for a total of 4 days. 2) Examine anaerobic media after 48 hours of incubation. - If there is no growth, discard the plates.
Culture Ø Growth on Blood agar, Chocolate agar and Mac. Conkey agar (or EMB) show a gram negative bacteria (Enterobacteriaceae and other gram negative bacilli ) Ø Growth only on Blood agar, Chocolate agar show a gram positive bacteria Ø Only growth on Chocolate agar show a fastidious bacteria Ø Identification: Must be done based on biochemical test based on kind of bacteria growth and gram smear
Culture - Consider organisms that grow aerobically and anaerobically as aerobes (facultative) - Consider organisms that grow only on the anaerobic BAP as obligate anaerobes. - Unfortunately, our laboratories do not have anaerobic organism identification capability. - Fungi could be isolated. - Correlate culture findings with the direct specimen Gram’s stain results.

Isolation of suspected contaminants Ø Environmental contamination is suspected when: a. Growth occurs outside the streak lines b. Growth occurs on the plates but not in the broth Ø Skin contamination is suspected when an organism normally present on the skin is isolated e. g. Coagulase-negative staphylococci, diptheroids

Result reporting: Report Gram stain, KOH, and AFS finding as an initial report. Report the isolated pathogen and its sensitivity pattern as a final report. Ø Negative cultures – report “No growth after 4 days”
Result reporting: (Positive cultures) 1) Preliminary report a. Issue when significant growth occurs b. Provide as much detail as possible 1 - Quantity and gram-morphology. Example: Moderate lactose-fermenting gram-negative rods 2 - Give organism name if known based upon rapid tests such as catalase, oxidase and spot indole Example 1: Many Staphylococcus aureus. Susceptibilities to follow. Example 2: Moderate Pseudomonas aeruginosa. Susceptibilities to follow.
Result reporting: (Positive cultures) 2) Final report Ø Report complete organism name (genus and species) – do not abbreviate Ø Report all appropriate susceptibility results (when applicable) Anaerobic bacteria - Report anaerobic nature and Gram’s stain morphology Example: Few anaerobic gram-positive cocci
Limitations A. False-positive cultures can occur due to contamination of the sample with skin flora. B. - False-negative cultures can occur due to: low numbers of organisms in specimen Prior antimicrobial treatment Fastidious nature of etiologic agent.

Thank you
- Slides: 69