Stereoisomerism Cistrans isomerism Chirality Enantiomer Meso Achiral Optical
















- Slides: 66

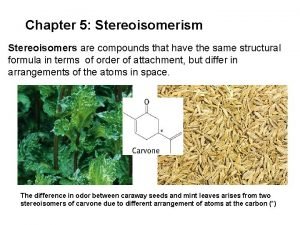
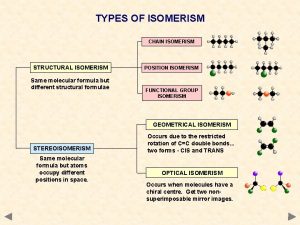
Stereoisomerism • • Cis/trans isomerism Chirality, Enantiomer Meso; Achiral Optical activity Haworth projection Fisher projection Three dimensional structure is critical in biochemistry 5 -1

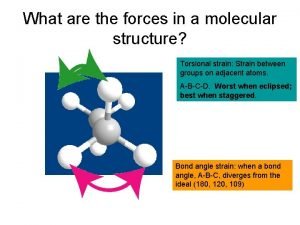
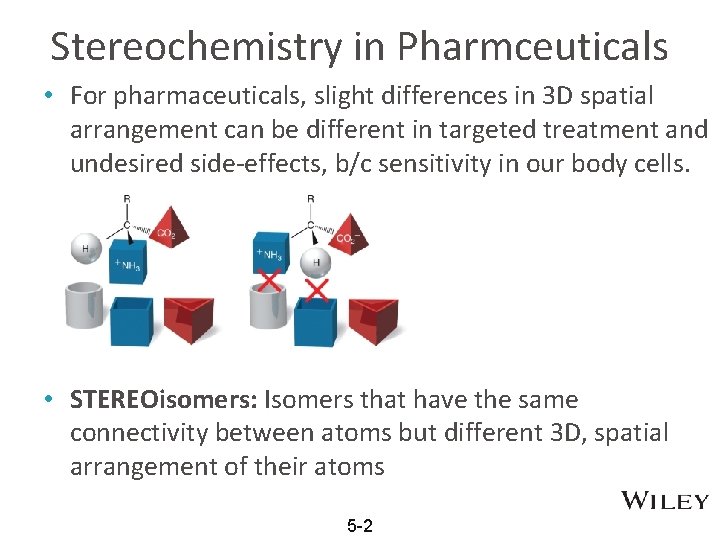
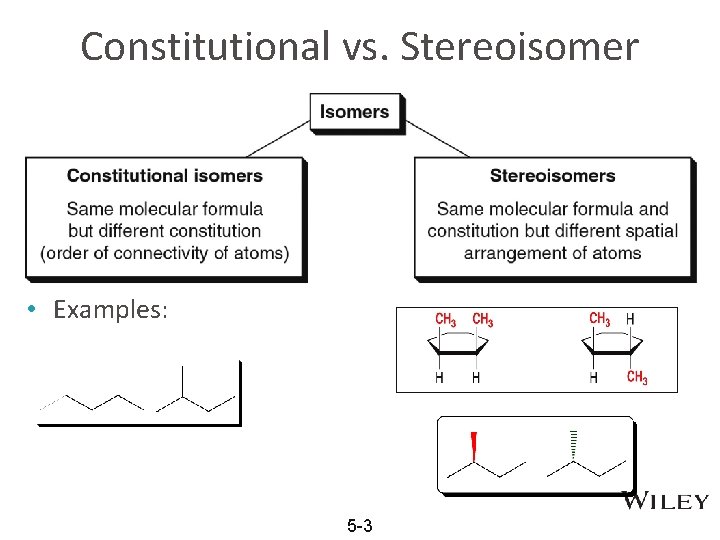
Stereochemistry in Pharmceuticals • For pharmaceuticals, slight differences in 3 D spatial arrangement can be different in targeted treatment and undesired side-effects, b/c sensitivity in our body cells. • STEREOisomers: Isomers that have the same connectivity between atoms but different 3 D, spatial arrangement of their atoms 5 -2

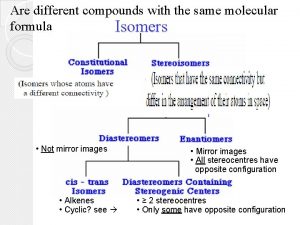
Constitutional vs. Stereoisomer • Examples: 5 -3

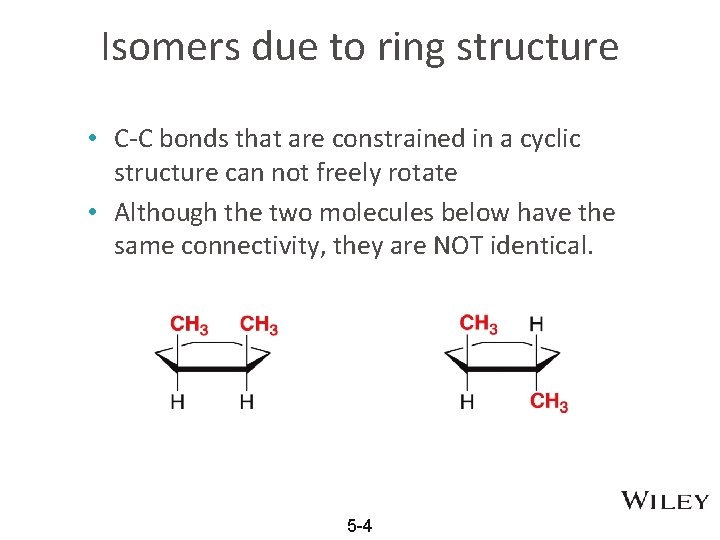
Isomers due to ring structure • C-C bonds that are constrained in a cyclic structure can not freely rotate • Although the two molecules below have the same connectivity, they are NOT identical. 5 -4

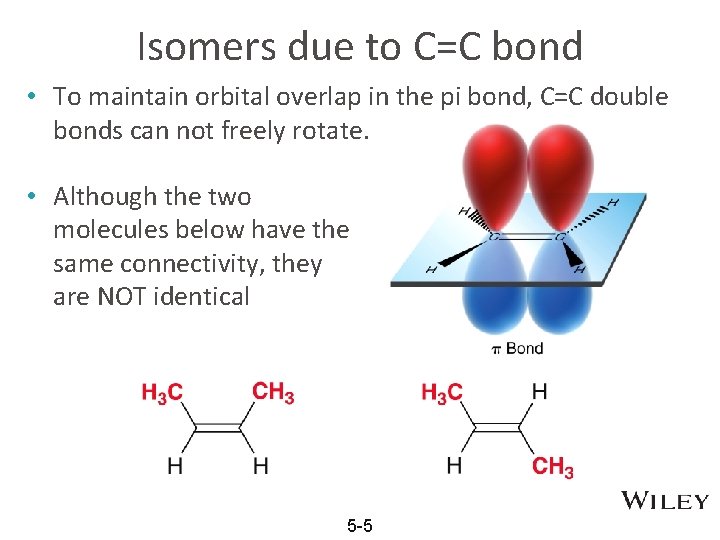
Isomers due to C=C bond • To maintain orbital overlap in the pi bond, C=C double bonds can not freely rotate. • Although the two molecules below have the same connectivity, they are NOT identical 5 -5

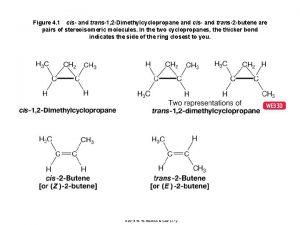
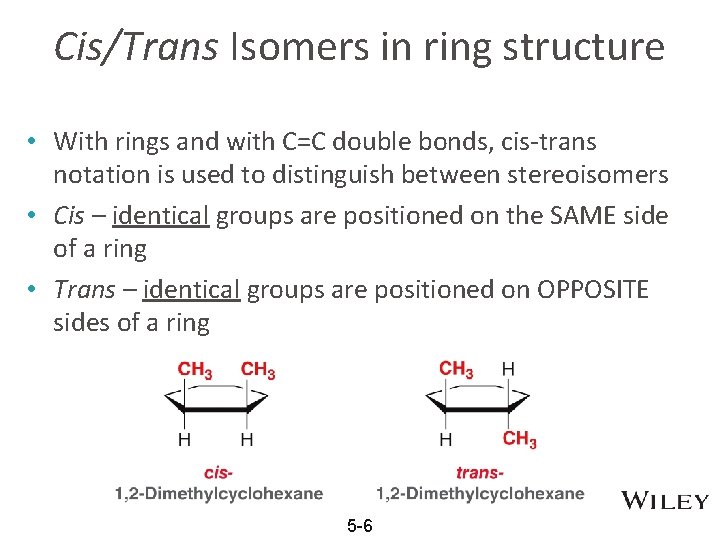
Cis/Trans Isomers in ring structure • With rings and with C=C double bonds, cis-trans notation is used to distinguish between stereoisomers • Cis – identical groups are positioned on the SAME side of a ring • Trans – identical groups are positioned on OPPOSITE sides of a ring 5 -6

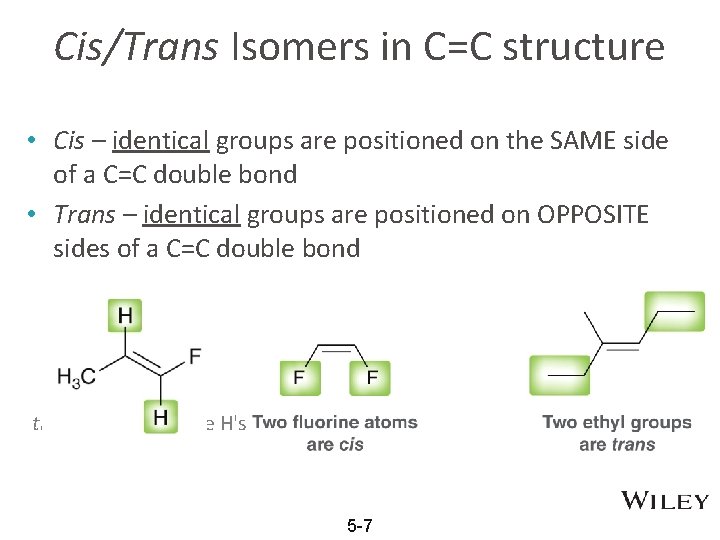
Cis/Trans Isomers in C=C structure • Cis – identical groups are positioned on the SAME side of a C=C double bond • Trans – identical groups are positioned on OPPOSITE sides of a C=C double bond trans because of the H's 5 -7

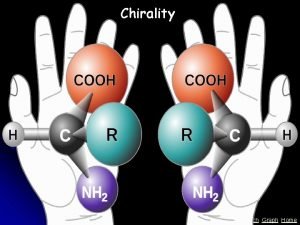
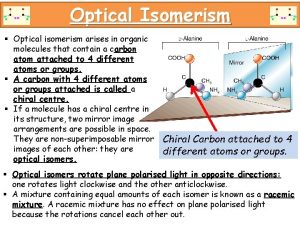
Chiral Stereoisomers • A chiral object is NOT identical to its mirror image, example: Right hand vs. Left hand • Many biomolecules are chiral: amino acid, glucose, enzyme. Our body can only digest glucose but not its mirror image, same way can only use L-amino acid but not the mirror image. • The thalidomide catastrophe 5 -8

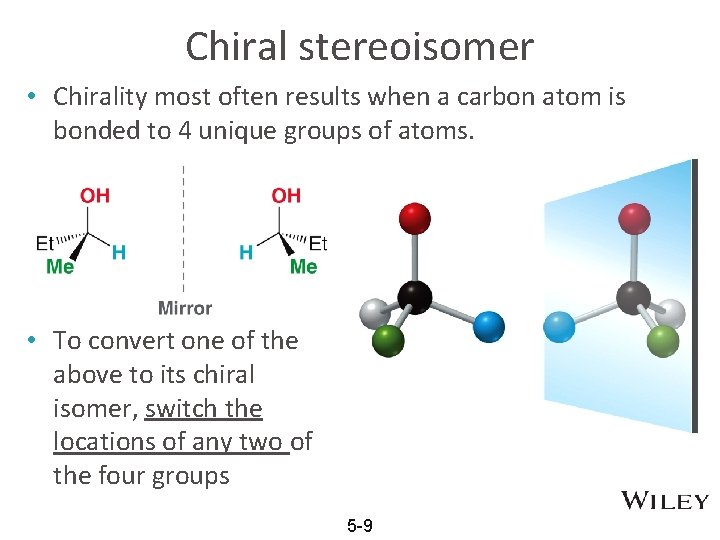
Chiral stereoisomer • Chirality most often results when a carbon atom is bonded to 4 unique groups of atoms. • To convert one of the above to its chiral isomer, switch the locations of any two of the four groups 5 -9

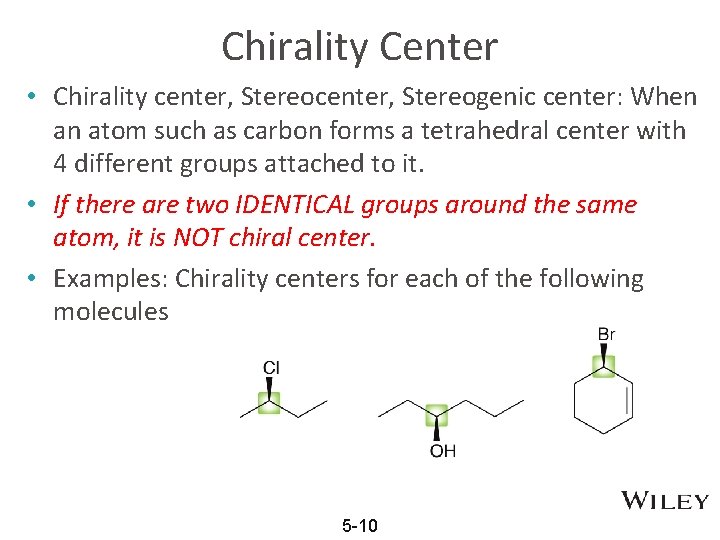
Chirality Center • Chirality center, Stereogenic center: When an atom such as carbon forms a tetrahedral center with 4 different groups attached to it. • If there are two IDENTICAL groups around the same atom, it is NOT chiral center. • Examples: Chirality centers for each of the following molecules 5 -10

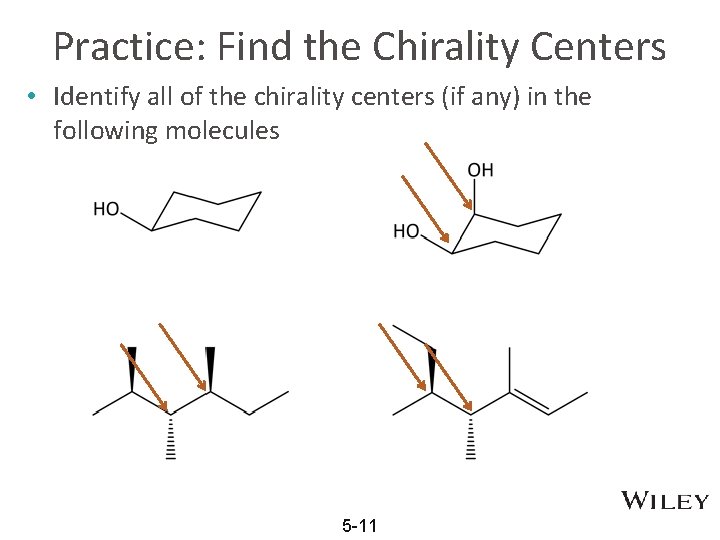
Practice: Find the Chirality Centers • Identify all of the chirality centers (if any) in the following molecules 5 -11

Enantiomers • Enantiomers are mirror image to each other, but are NONidentical and NONsuperimposable: Odor difference: Living beings such as human, animal, and plants are made of chiral molecules, thus respond differently to chiral molecule 5 -12

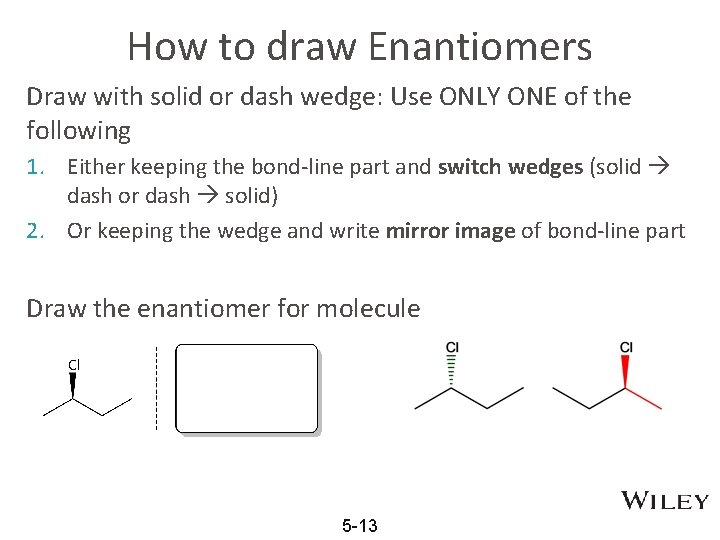
How to draw Enantiomers Draw with solid or dash wedge: Use ONLY ONE of the following 1. Either keeping the bond-line part and switch wedges (solid dash or dash solid) 2. Or keeping the wedge and write mirror image of bond-line part Draw the enantiomer for molecule 5 -13

Naming Enantiomers • The Cahn, Ingold and Prelog system 1. Using atomic numbers, prioritize the 4 groups attached to the chirality center 2. Arrange the molecule in space so the lowest priority group faces away from you 3. Count the group priorities 1… 2… 3 to determine whether the order progresses in a clockwise or counterclockwise direction 4. Clockwise = R and Counterclockwise = S 5 -14

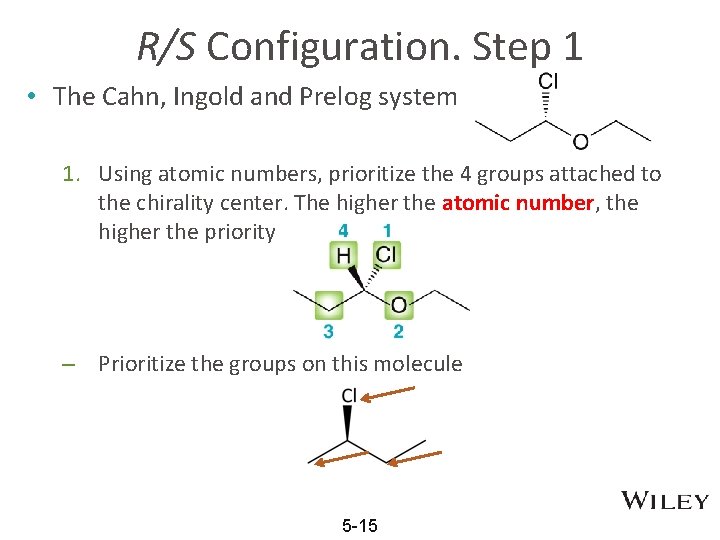
R/S Configuration. Step 1 • The Cahn, Ingold and Prelog system 1. Using atomic numbers, prioritize the 4 groups attached to the chirality center. The higher the atomic number, the higher the priority – Prioritize the groups on this molecule 5 -15

R/S Configuration. Step 2 2. Arrange the molecule in space so the lowest priority group faces away from you. Rotate the molecule if the lowest priority group is NOT away from you. – Shortcut? The Double switch: Switch two different pairs of groups – Selfpractice with models: from hand to mind. – More help? Link: https: //www. khanacademy. org/science/organic-chemistry/stereochemistry-topic/chirality-r-s-system/v/r-s--cahn-ingold-prelog--naming-system-example-2 5 -16

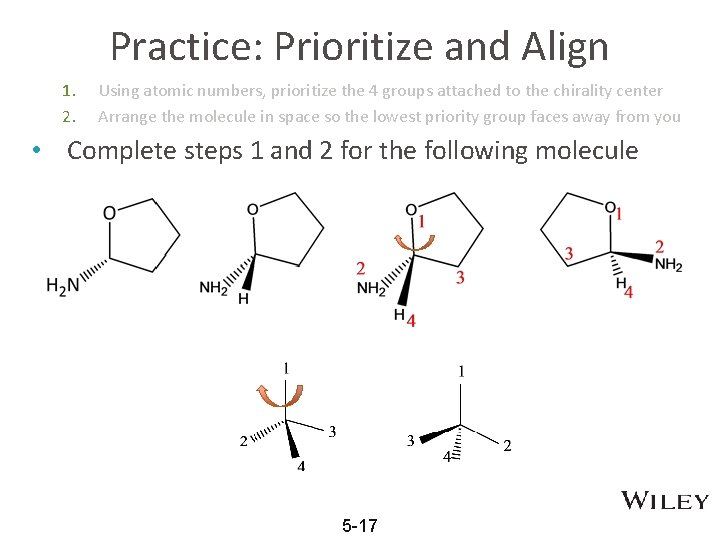
Practice: Prioritize and Align 1. 2. Using atomic numbers, prioritize the 4 groups attached to the chirality center Arrange the molecule in space so the lowest priority group faces away from you • Complete steps 1 and 2 for the following molecule 5 -17

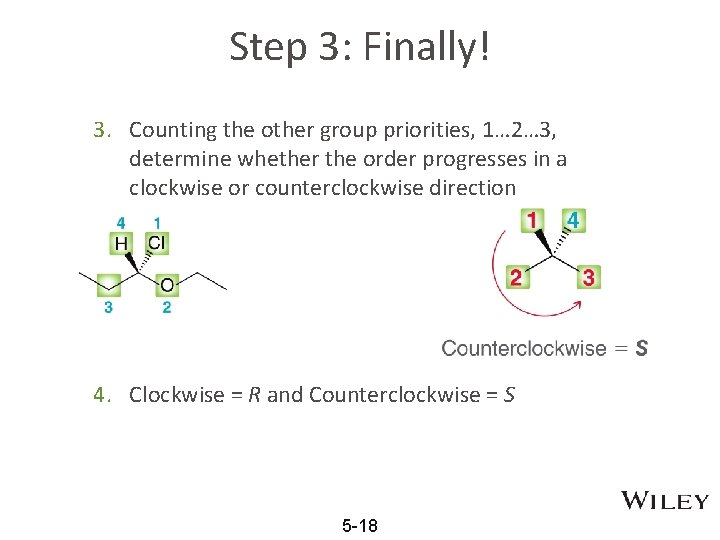
Step 3: Finally! 3. Counting the other group priorities, 1… 2… 3, determine whether the order progresses in a clockwise or counterclockwise direction 4. Clockwise = R and Counterclockwise = S 5 -18

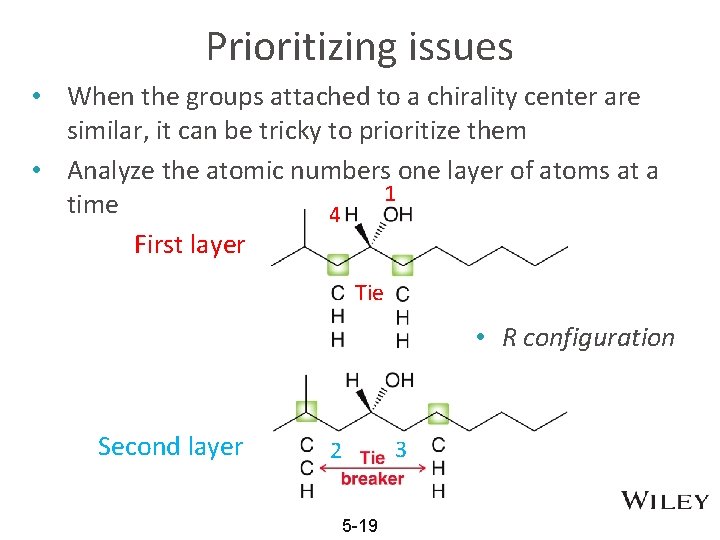
Prioritizing issues • When the groups attached to a chirality center are similar, it can be tricky to prioritize them • Analyze the atomic numbers one layer of atoms at a 1 time 4 First layer Tie • R configuration Second layer 3 2 5 -19

One layer a time • Analyze the atomic numbers one layer of atoms at a 4 1 time • The priority is • First layer based on the first Tie • Second layer 3 2 5 -20 point of difference, NOT the sum of the atomic numbers • R again!

Prioritize double bond • When prioritizing for the Cahn, Ingold and Prelog system, double bonds count as two single bonds • Determine R or S for the following molecule. • R! 5 -21

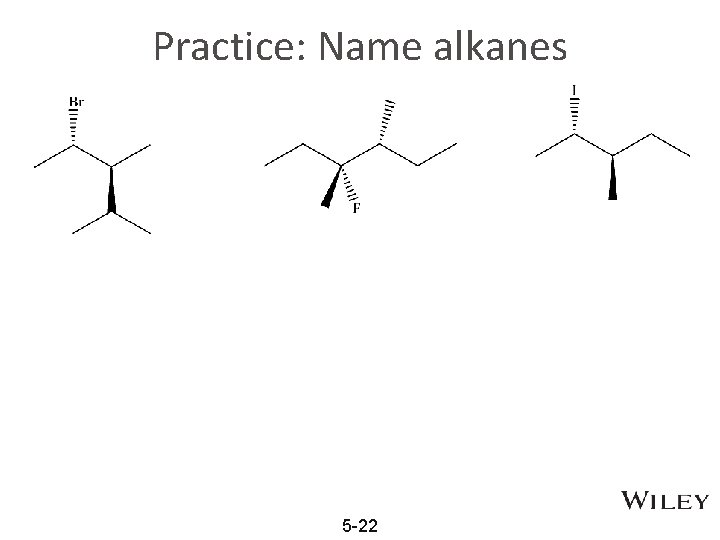
Practice: Name alkanes 5 -22

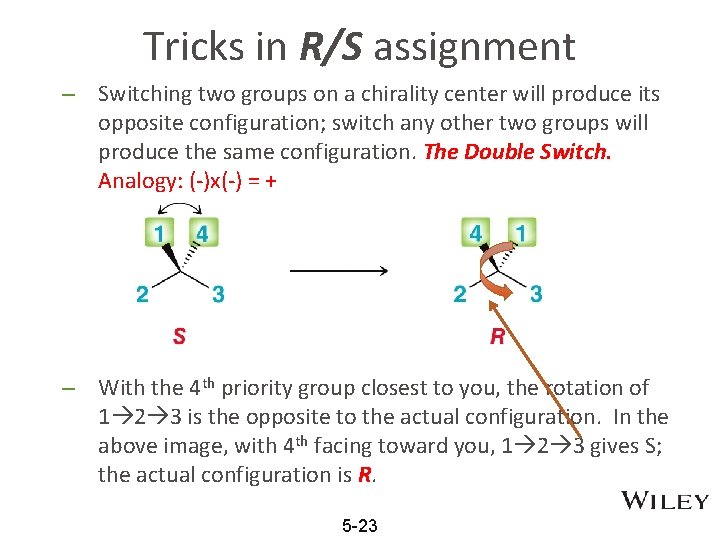
Tricks in R/S assignment – Switching two groups on a chirality center will produce its opposite configuration; switch any other two groups will produce the same configuration. The Double Switch. Analogy: (-)x(-) = + – With the 4 th priority group closest to you, the rotation of 1 2 3 is the opposite to the actual configuration. In the above image, with 4 th facing toward you, 1 2 3 gives S; the actual configuration is R. 5 -23

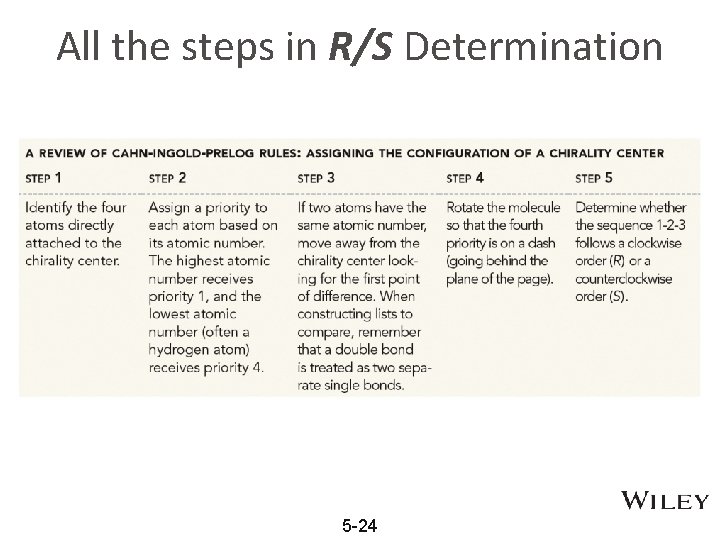
All the steps in R/S Determination 5 -24

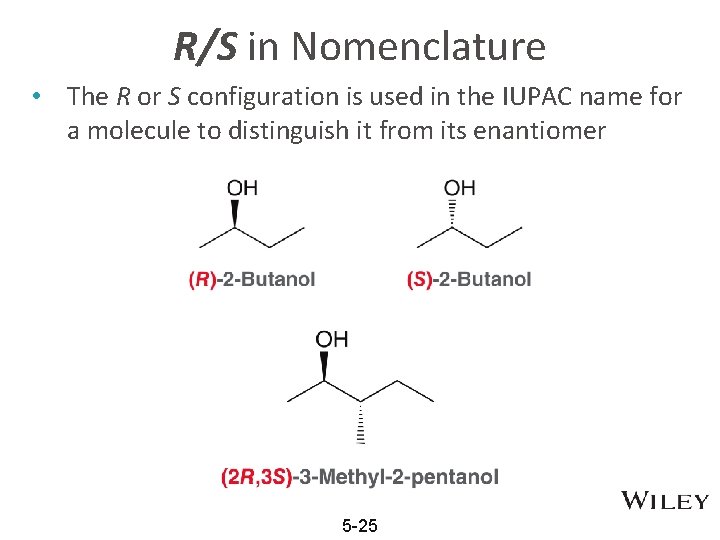
R/S in Nomenclature • The R or S configuration is used in the IUPAC name for a molecule to distinguish it from its enantiomer 5 -25

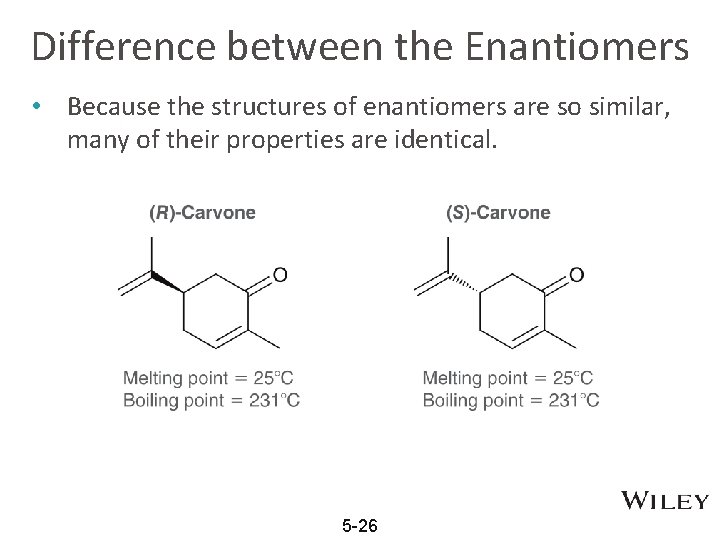
Difference between the Enantiomers • Because the structures of enantiomers are so similar, many of their properties are identical. 5 -26

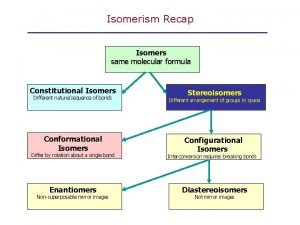
Recap: All about Isomers • Categories of isomers 5 -27

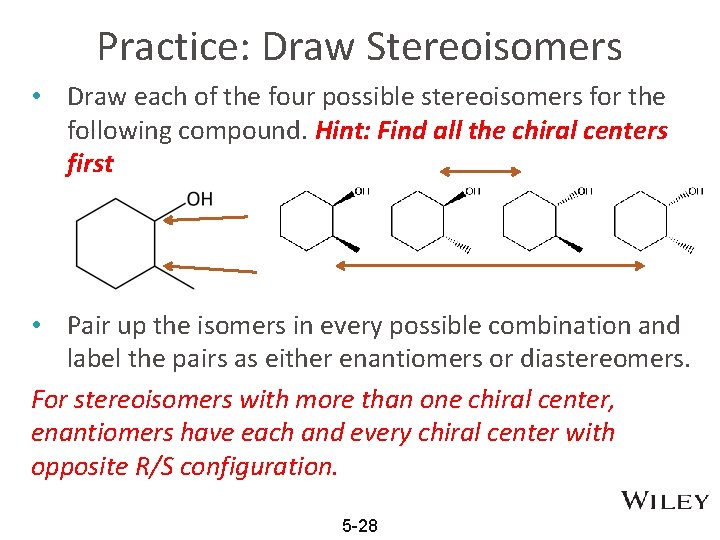
Practice: Draw Stereoisomers • Draw each of the four possible stereoisomers for the following compound. Hint: Find all the chiral centers first • Pair up the isomers in every possible combination and label the pairs as either enantiomers or diastereomers. For stereoisomers with more than one chiral center, enantiomers have each and every chiral center with opposite R/S configuration. 5 -28

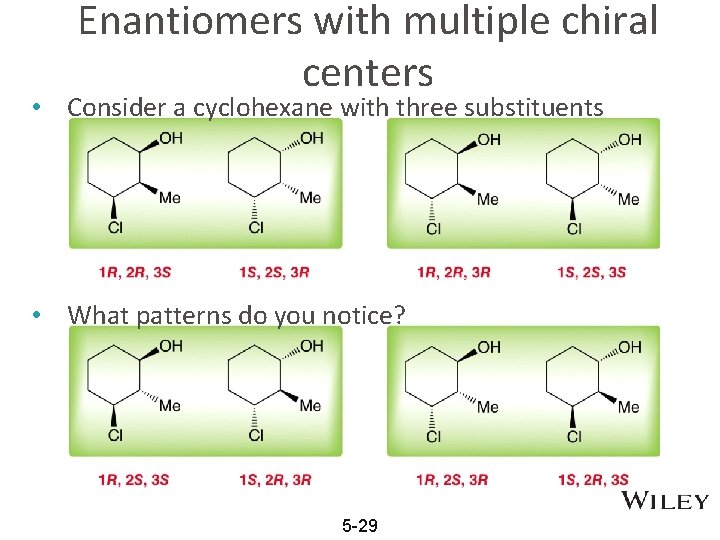
Enantiomers with multiple chiral centers • Consider a cyclohexane with three substituents • What patterns do you notice? 5 -29

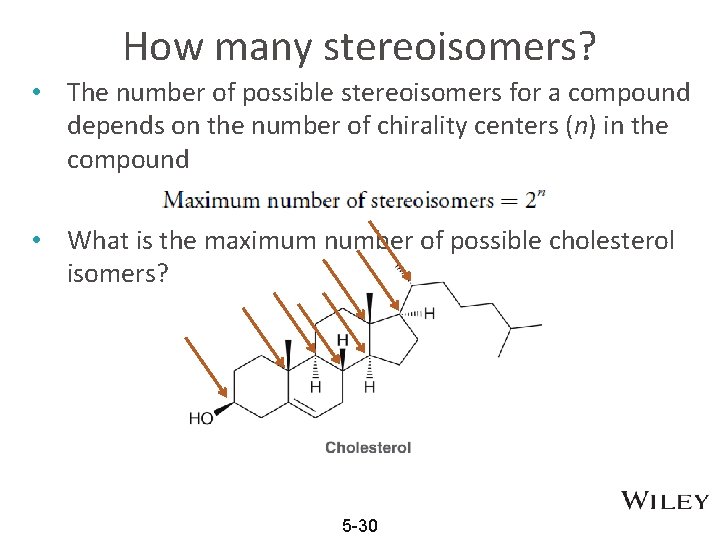
How many stereoisomers? • The number of possible stereoisomers for a compound depends on the number of chirality centers (n) in the compound • What is the maximum number of possible cholesterol isomers? 5 -30

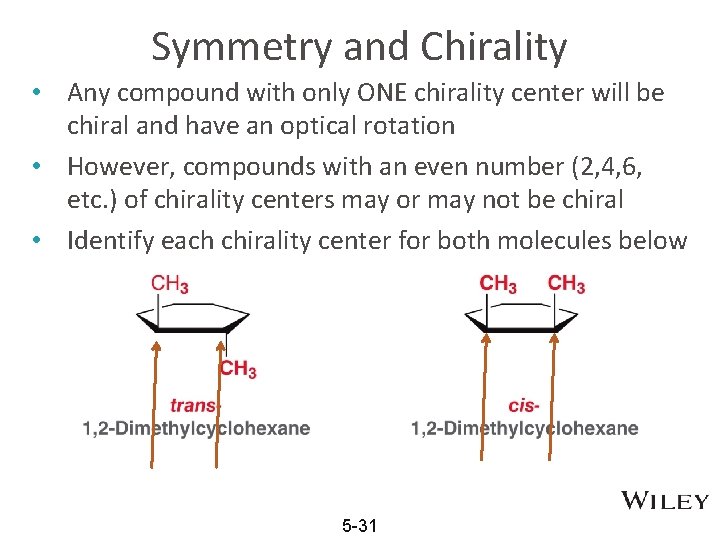
Symmetry and Chirality • Any compound with only ONE chirality center will be chiral and have an optical rotation • However, compounds with an even number (2, 4, 6, etc. ) of chirality centers may or may not be chiral • Identify each chirality center for both molecules below 5 -31

Plane of Symmetry, Achiral • Both molecules have 2 chirality centers, but only one is a chiral molecule, and the other is achiral • If a molecule has a plane of symmetry, it will be achiral • Half of the molecule reflects the other half • Its optical activity will be cancelled out within the molecule, similar to how a pair or mirror image enantiomers cancel out each others optical rotation 5 -32

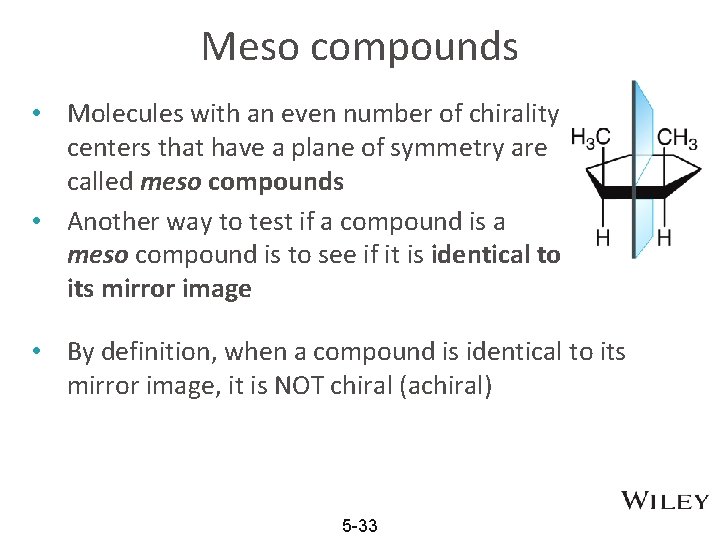
Meso compounds • Molecules with an even number of chirality centers that have a plane of symmetry are called meso compounds • Another way to test if a compound is a meso compound is to see if it is identical to its mirror image • By definition, when a compound is identical to its mirror image, it is NOT chiral (achiral) 5 -33

Symmetry and the n 2 rule • In another example, the plane of symmetry identifies it as a meso compound • meso compounds also have less than the predicted number of stereoisomers based on the 2(n) formula • Draw all four expected isomers and show two of them are identical. A handheld model might be helpful 5 -34

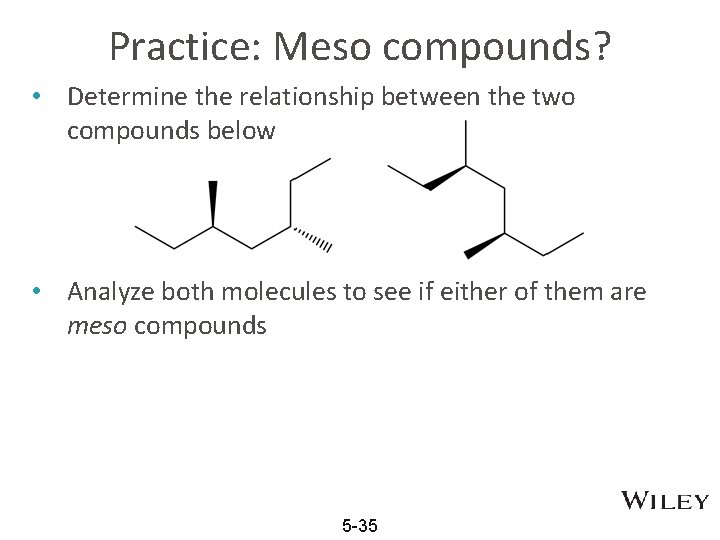
Practice: Meso compounds? • Determine the relationship between the two compounds below • Analyze both molecules to see if either of them are meso compounds 5 -35

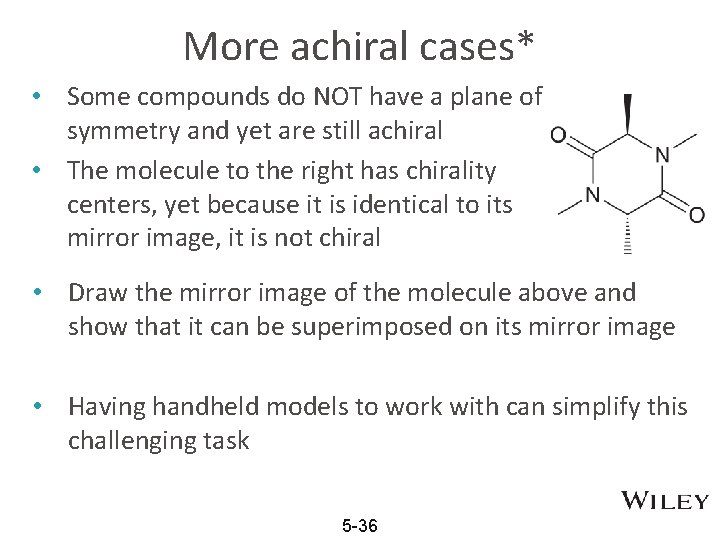
More achiral cases* • Some compounds do NOT have a plane of symmetry and yet are still achiral • The molecule to the right has chirality centers, yet because it is identical to its mirror image, it is not chiral • Draw the mirror image of the molecule above and show that it can be superimposed on its mirror image • Having handheld models to work with can simplify this challenging task 5 -36

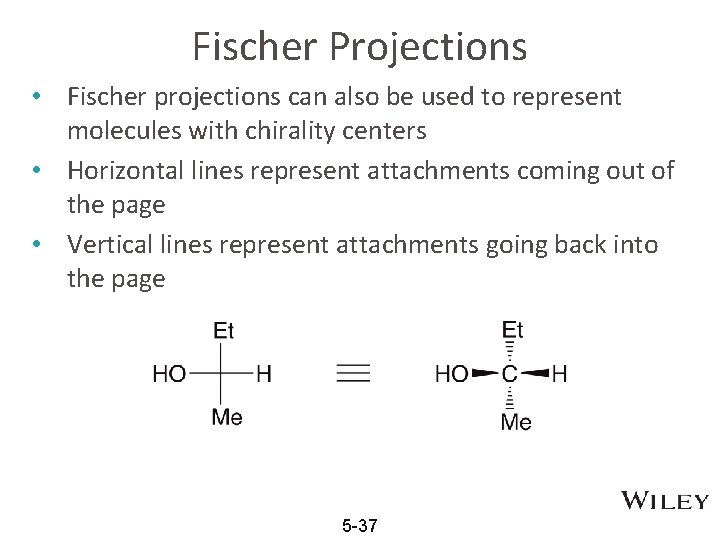
Fischer Projections • Fischer projections can also be used to represent molecules with chirality centers • Horizontal lines represent attachments coming out of the page • Vertical lines represent attachments going back into the page 5 -37

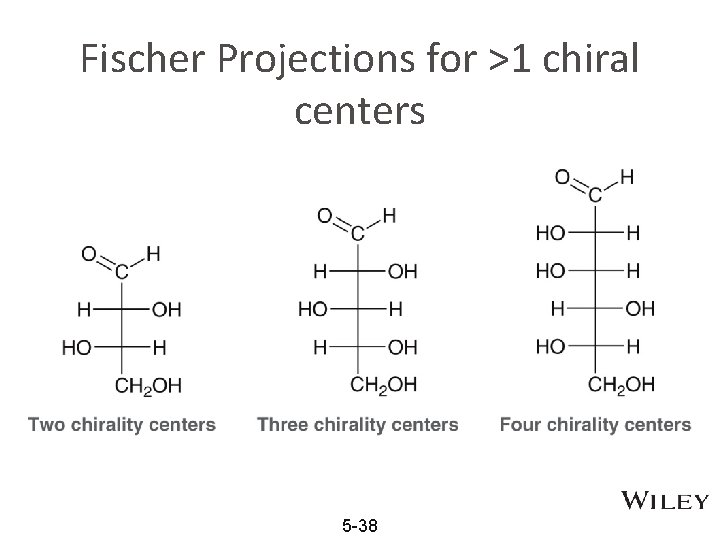
Fischer Projections for >1 chiral centers 5 -38

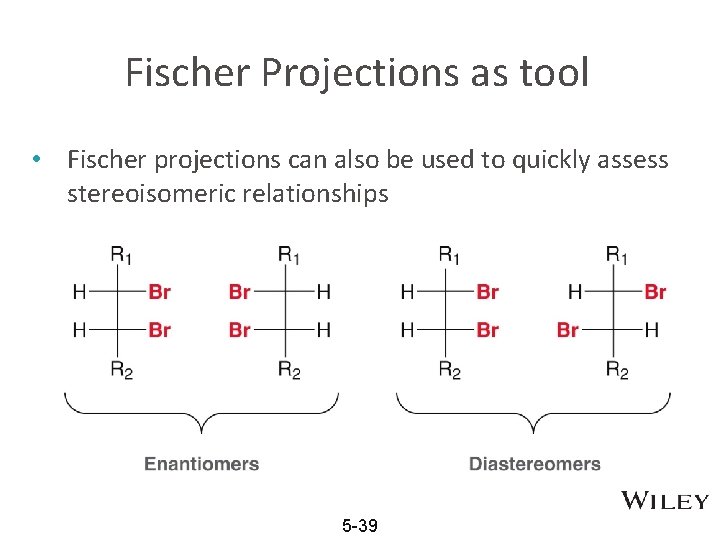
Fischer Projections as tool • Fischer projections can also be used to quickly assess stereoisomeric relationships 5 -39

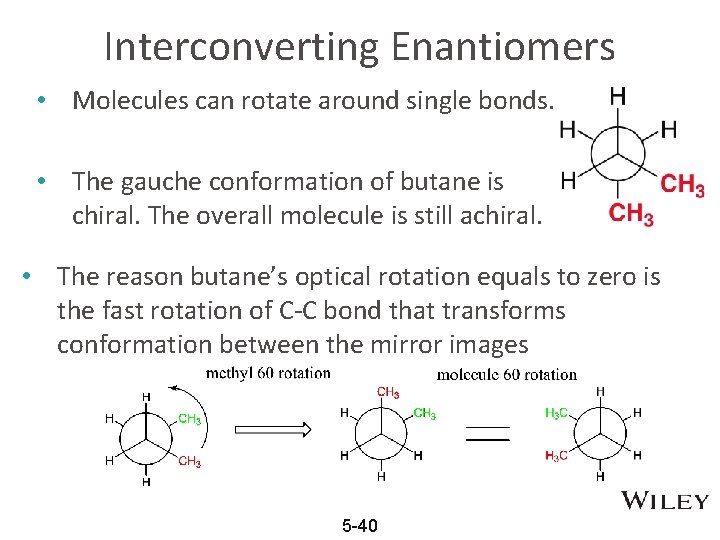
Interconverting Enantiomers • Molecules can rotate around single bonds. • The gauche conformation of butane is chiral. The overall molecule is still achiral. • The reason butane’s optical rotation equals to zero is the fast rotation of C-C bond that transforms conformation between the mirror images 5 -40

Substituted Cyclohexane • Chair conformation vs. Haworth projection • The Haworth image (symmetrical) shows the compound as an achiral meso compound, although Chair conformation does not display a plane of symmetry • Due to the quick intramolecular bond rotation, Haworth projection ~ “Average” structure 5 -41

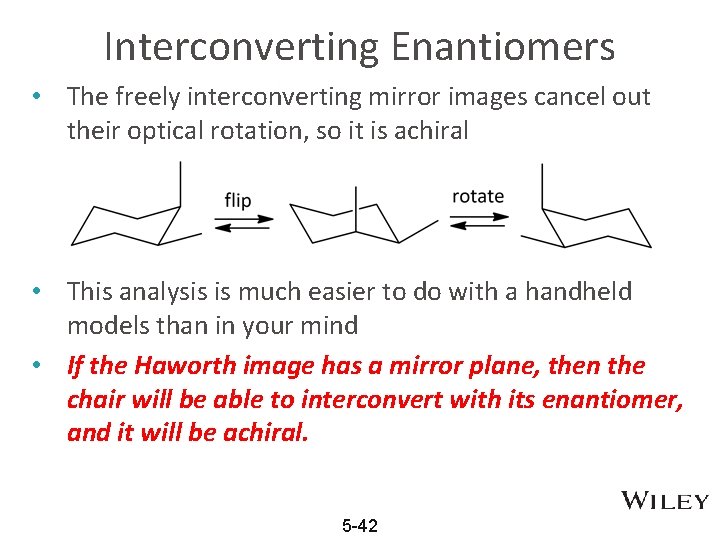
Interconverting Enantiomers • The freely interconverting mirror images cancel out their optical rotation, so it is achiral • This analysis is much easier to do with a handheld models than in your mind • If the Haworth image has a mirror plane, then the chair will be able to interconvert with its enantiomer, and it will be achiral. 5 -42

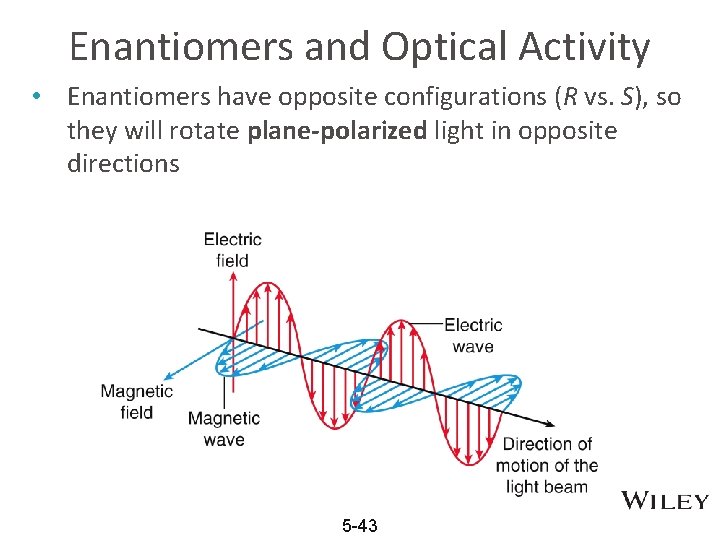
Enantiomers and Optical Activity • Enantiomers have opposite configurations (R vs. S), so they will rotate plane-polarized light in opposite directions 5 -43

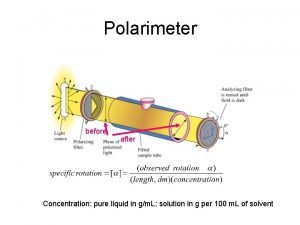
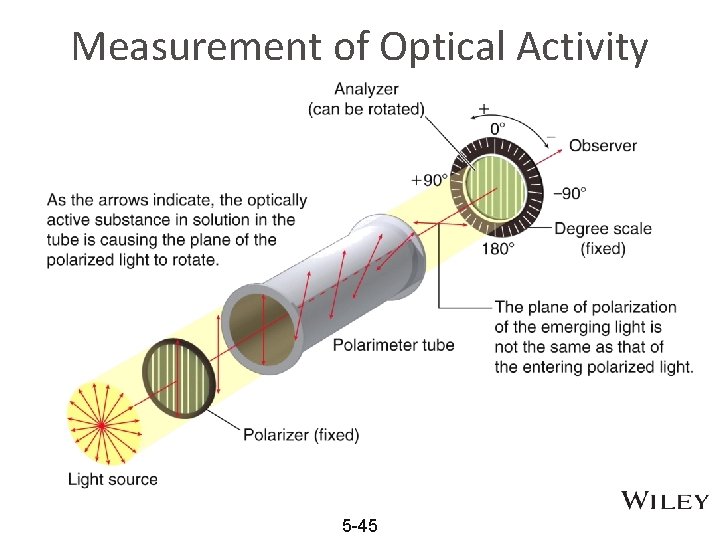
Chiral compounds are optically active • To get light waves that travel in only one plane (polarized light), light travels through a filter (such as in Polaroid sunglasses) • When plane polarized light is directed through a sample of a pure chiral compound, the plane that the light travels on will rotate. • Compounds that can rotate the plane or planepolarized light are called optically active 5 -44

Measurement of Optical Activity 5 -45

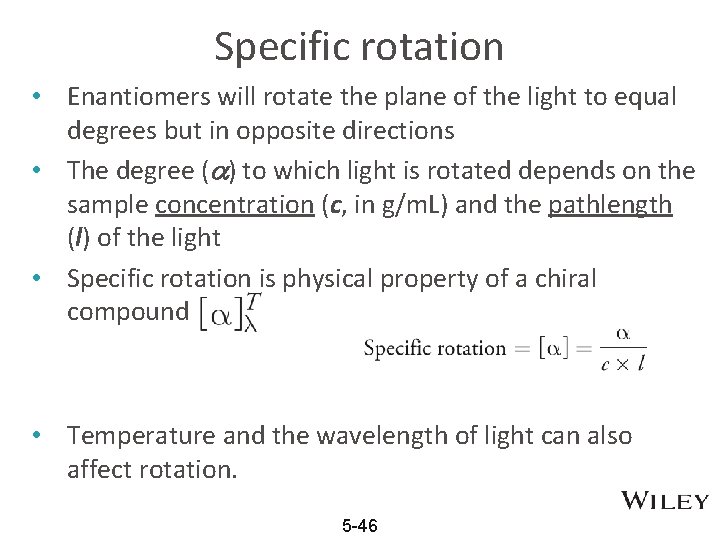
Specific rotation • Enantiomers will rotate the plane of the light to equal degrees but in opposite directions • The degree ( ) to which light is rotated depends on the sample concentration (c, in g/m. L) and the pathlength (l) of the light • Specific rotation is physical property of a chiral compound • Temperature and the wavelength of light can also affect rotation. 5 -46

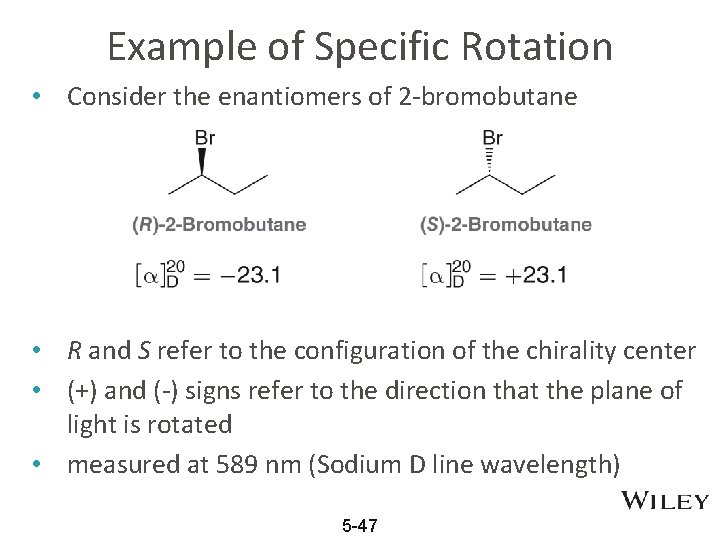
Example of Specific Rotation • Consider the enantiomers of 2 -bromobutane • R and S refer to the configuration of the chirality center • (+) and (-) signs refer to the direction that the plane of light is rotated • measured at 589 nm (Sodium D line wavelength) 5 -47

Optical Activity vs. R/S • Direction of light rotation (+/-) can not be predicted by R/S configuration Example: • L-aspartic acid: levorotatory (-) at 20°C, dextrorotatory (+) at 100°C, it is 5 -48

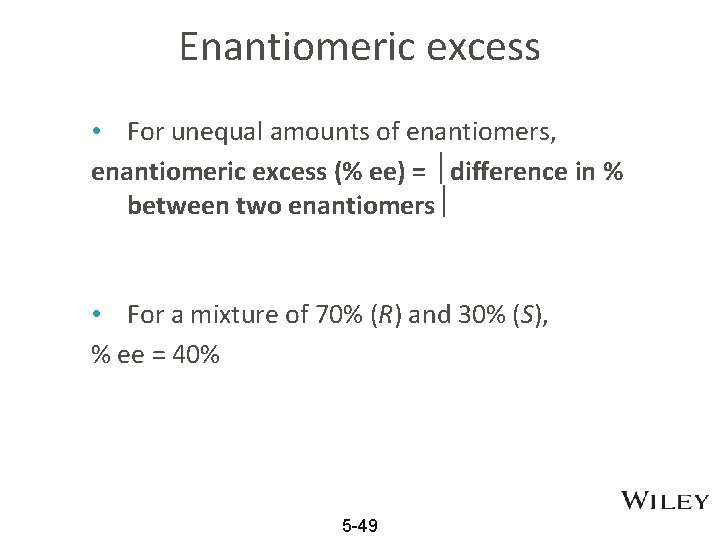
Enantiomeric excess • For unequal amounts of enantiomers, enantiomeric excess (% ee) = difference in % between two enantiomers • For a mixture of 70% (R) and 30% (S), % ee = 40% 5 -49

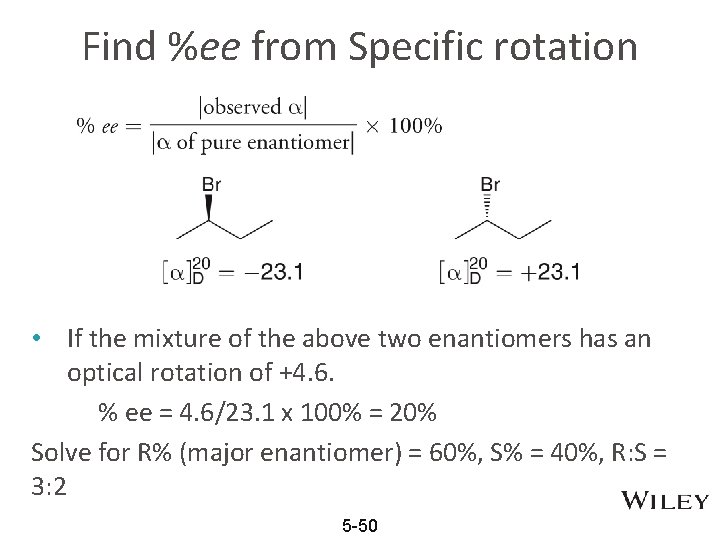
Find %ee from Specific rotation • If the mixture of the above two enantiomers has an optical rotation of +4. 6. % ee = 4. 6/23. 1 x 100% = 20% Solve for R% (major enantiomer) = 60%, S% = 40%, R: S = 3: 2 5 -50

Resolution of Enantiomers • Most methods of separating compounds from one another take advantages of the compounds’ different physical properties – Distillation – separates compounds with different boiling points – Recrystallization – separates compounds with different solubilities – Can you think of more methods of separation or purification? • Such methods often don’t work to separate one enantiomer from its partner. 5 -51

Pasteur’s Solution • In 1847, Pasteur performed the first resolution of enantiomers from a racemic mixture of tartaric acid salts • The different enantiomers formed different shaped crystals that were separated by hand using tweezers • This method doesn’t work for most pairs of enantiomers. 5 -52

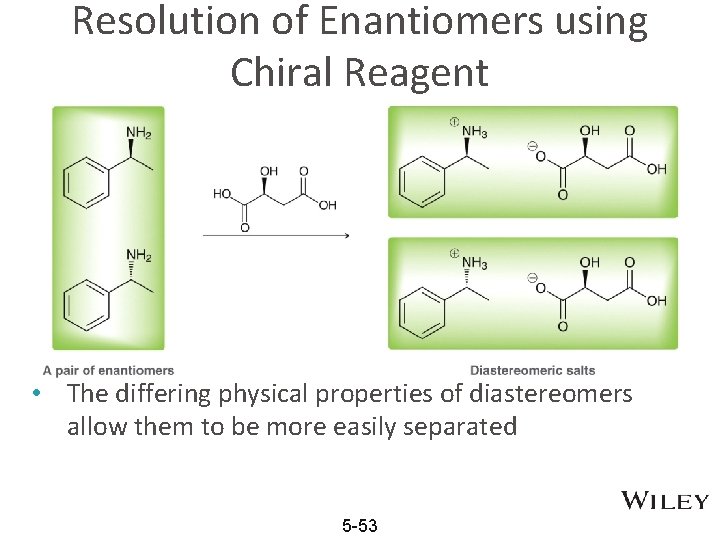
Resolution of Enantiomers using Chiral Reagent • The differing physical properties of diastereomers allow them to be more easily separated 5 -53

Resolution of Enantiomers using Chromatography • Affinity chromatography is often used to separate enantiomers • For example, a glass column or tube can be packed with particles of a chiral compound as stationary phase • Mixture of enantiomers will have different affinity (IMF) toward the chiral stationary phase, therefore the enantiomers can be separated 5 -54

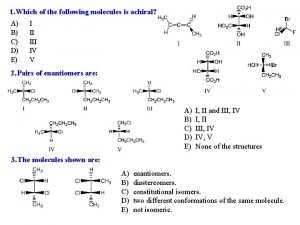
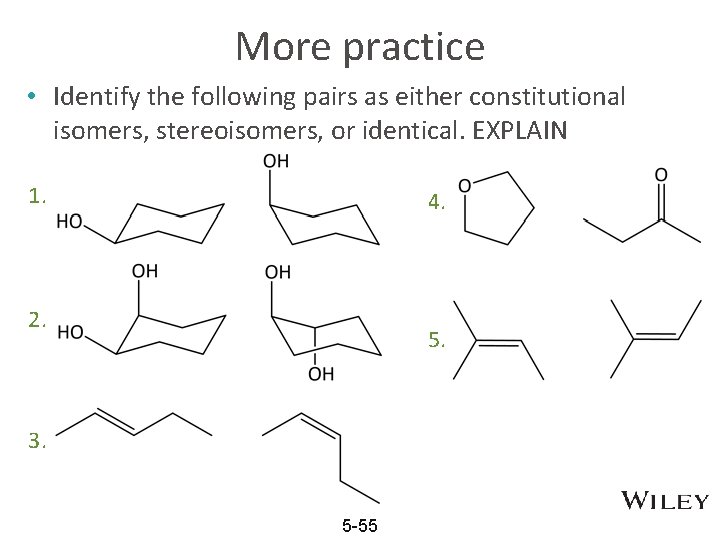
More practice • Identify the following pairs as either constitutional isomers, stereoisomers, or identical. EXPLAIN 1. 4. 2. 5. 3. 5 -55

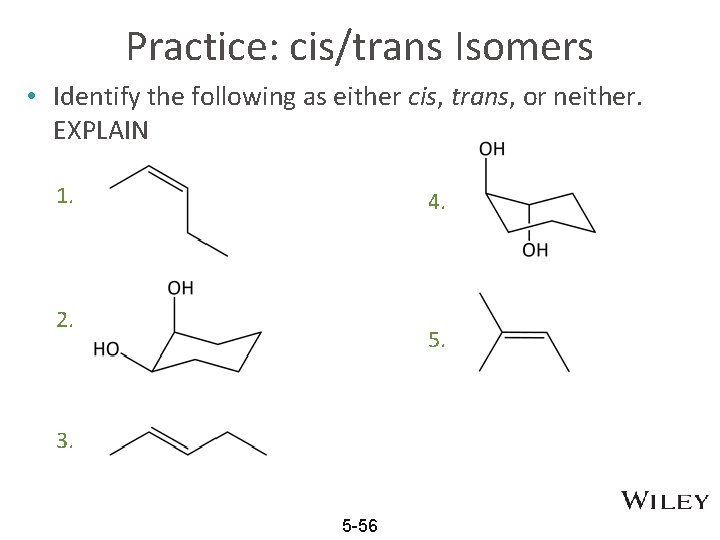
Practice: cis/trans Isomers • Identify the following as either cis, trans, or neither. EXPLAIN 1. 4. 2. 5. 3. 5 -56

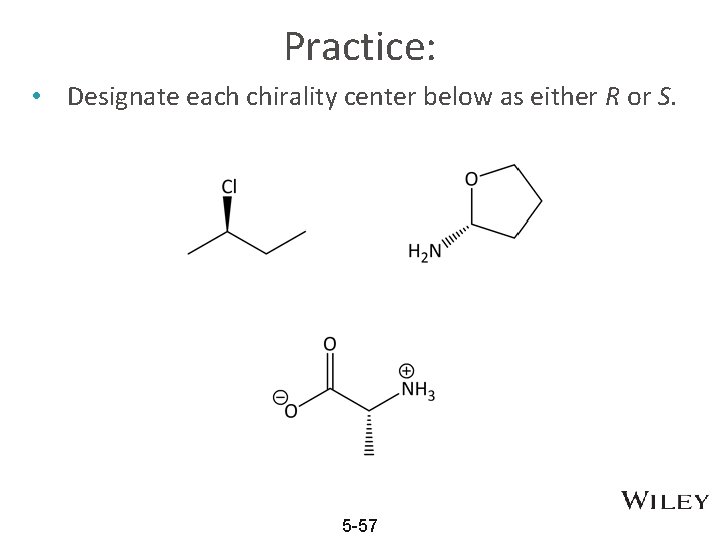
Practice: • Designate each chirality center below as either R or S. 5 -57

Additional Practice Problems • Draw a pair of constitutional isomers. EXPLAIN 5 -58

Additional Practice Problems • Draw a pair of cis-trans isomers. EXPLAIN 5 -59

Additional Practice Problems • Draw a pair of diastereomers that are not cis-trans isomers. EXPLAIN 5 -60

Additional Practice Problems • Draw a pair of enantiomers. Explain 5 -61

Additional Practice Problems • Draw a molecule with 3 chiral centers. 5 -62

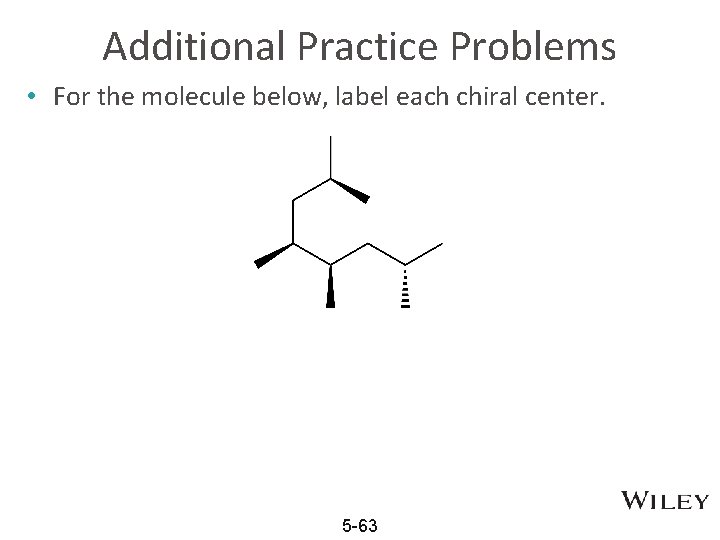
Additional Practice Problems • For the molecule below, label each chiral center. 5 -63

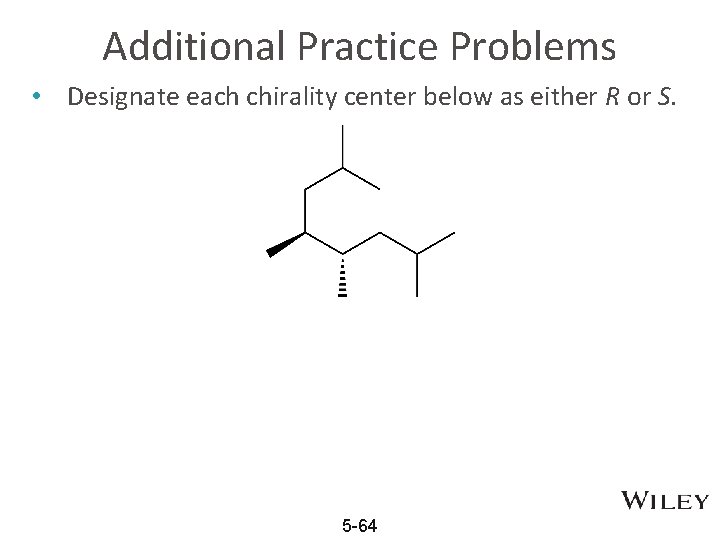
Additional Practice Problems • Designate each chirality center below as either R or S. 5 -64

Additional Practice Problems • If pure R enantiomer has a specific rotation of +33 degrees, what will the rotation be when you have a mixture with a R/S ratio = 44/56? 5 -65

Additional Practice Problems • Determine the relationship between the pairs below. 5 -66
 Optical isomers of octahedral complexes
Optical isomers of octahedral complexes R and s isomers
R and s isomers Thirosine
Thirosine 2-hydroxypropanenitrile displays optical isomerism
2-hydroxypropanenitrile displays optical isomerism Chirality
Chirality Early man
Early man Chiral and achiral meaning
Chiral and achiral meaning Chirality
Chirality Constitutional isomer vs stereoisomer
Constitutional isomer vs stereoisomer Structural geometric and enantiomer isomers
Structural geometric and enantiomer isomers Gambarkan isomer r dan s dari 3 fenil 2 butanol
Gambarkan isomer r dan s dari 3 fenil 2 butanol Chirality
Chirality Chirality centers in sucralose
Chirality centers in sucralose C khiral
C khiral Propane conformers
Propane conformers Structural isomers
Structural isomers L and d isomers
L and d isomers Polymerization isomerism
Polymerization isomerism Isomers of c7 h16
Isomers of c7 h16 Unsaturated compounds examples
Unsaturated compounds examples Geometrical isomerism
Geometrical isomerism Coordination outline
Coordination outline Priority of functional groups
Priority of functional groups Hexaldose
Hexaldose Introduction to stereochemistry
Introduction to stereochemistry Chiral and achiral carbon
Chiral and achiral carbon Chiral in greek
Chiral in greek Which of the following molecules are achiral?
Which of the following molecules are achiral? Salamureno meso
Salamureno meso The rituals to fortify african babies against evil
The rituals to fortify african babies against evil Gotova jela u konzervi
Gotova jela u konzervi Holo proto meso tele
Holo proto meso tele Micro level
Micro level Secondo tono cardiaco
Secondo tono cardiaco Mikro makro- meso perspektiv
Mikro makro- meso perspektiv 2,3-dichlorobutane
2,3-dichlorobutane Gemaksgoederen voorbeelden
Gemaksgoederen voorbeelden Meso compound
Meso compound Calculate the specific rotation of glyceraldehyde
Calculate the specific rotation of glyceraldehyde Meso farmasi
Meso farmasi Ethische dilemma's sociaal werk
Ethische dilemma's sociaal werk Composé méso
Composé méso Svinjsko meso dijelovi
Svinjsko meso dijelovi Coanocito
Coanocito Meso farmasi
Meso farmasi Meso dalam farmasi adalah
Meso dalam farmasi adalah Milanska obrada svinje
Milanska obrada svinje Meso omgeving
Meso omgeving Makro mikro meso
Makro mikro meso Tropo strato meso thermo exo
Tropo strato meso thermo exo Kategorizacija mesa goveda
Kategorizacija mesa goveda 2-iodobutana
2-iodobutana Meso era shelter
Meso era shelter Meso compound
Meso compound Mesosternale
Mesosternale Protostomio
Protostomio Olmec leaders
Olmec leaders Meso compound
Meso compound Tropo strato meso thermo exo
Tropo strato meso thermo exo Kako konzervirati meso
Kako konzervirati meso Mesosystem
Mesosystem Pars condensa
Pars condensa Proto meso tele holo
Proto meso tele holo Meso esternal
Meso esternal Meso compound
Meso compound Optical purity formula
Optical purity formula Mesõ
Mesõ