Stereochemistry of Organic Compounds PART A Dr Suban

Stereochemistry of Organic Compounds (PART A) Dr Suban K Sahoo MSc, NET (JRF Qualified), Ph. D Reference Book: Organic Chemistry by Paula Yurkanis Bruice, 3 rd Edition
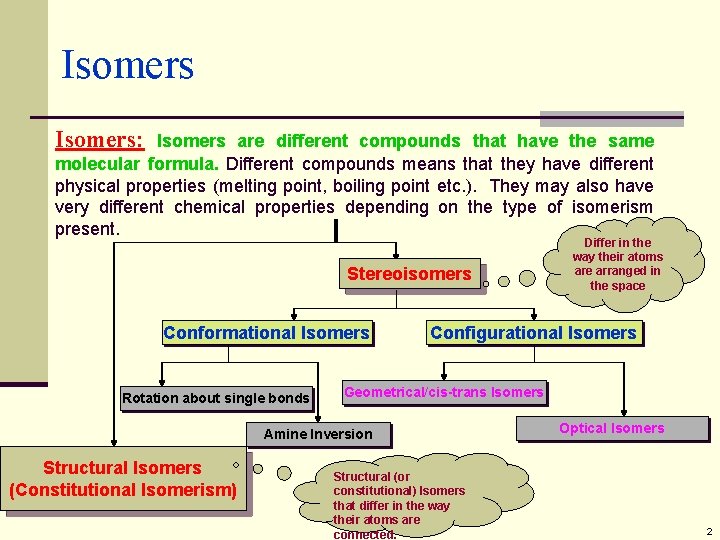
Isomers: Isomers are different compounds that have the same molecular formula. Different compounds means that they have different physical properties (melting point, boiling point etc. ). They may also have very different chemical properties depending on the type of isomerism present. Stereoisomers Conformational Isomers Rotation about single bonds Configurational Isomers Geometrical/cis-trans Isomers Amine Inversion Structural Isomers (Constitutional Isomerism) Differ in the way their atoms are arranged in the space Structural (or constitutional) Isomers that differ in the way their atoms are connected. Optical Isomers 2
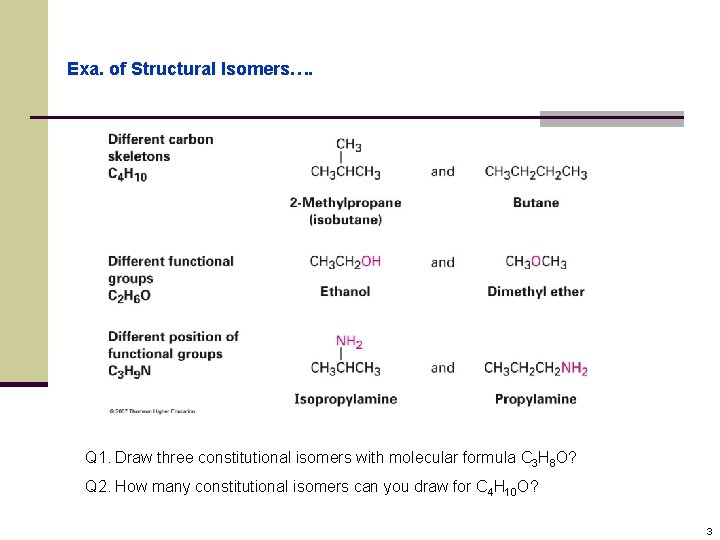
Exa. of Structural Isomers…. Q 1. Draw three constitutional isomers with molecular formula C 3 H 8 O? Q 2. How many constitutional isomers can you draw for C 4 H 10 O? 3

Conformations of Acyclic Alkanes Conformations are different arrangements of atoms that are interconverted by rotation about single bonds. Conformers can be represented in two different types : Views a C-C bond from an oblique angle Views a C-C bond from front to back 4

Types of conformers…. We do not observe perfectly free rotation. There is a barrier to rotation, and some conformers are more stable than others; based on this observations, conformers are of (A) Staggered : A low energy conformation where the bonds on adjacent atoms bisect each other (60 o dihedral angle), maximizing the separation. (B) Eclipsed : A high energy conformation where the bonds on adjacent atoms are aligned with each other (0 o dihedral angle). Example 1…. . Ethane Conformers 5
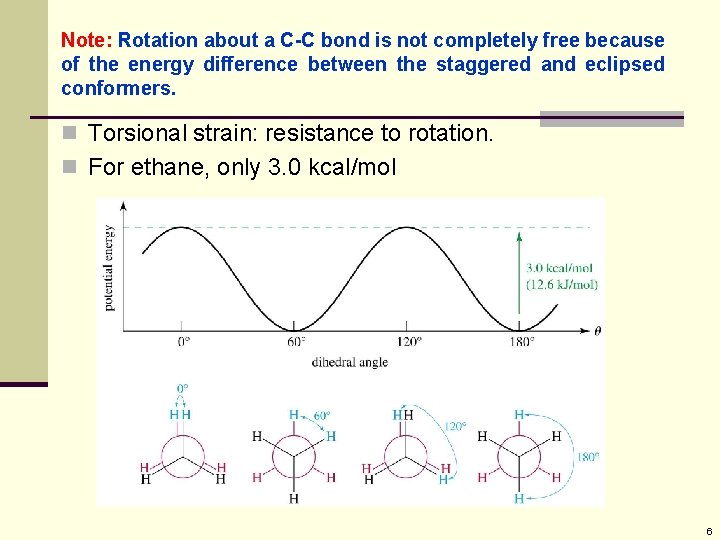
Note: Rotation about a C-C bond is not completely free because of the energy difference between the staggered and eclipsed conformers. n Torsional strain: resistance to rotation. n For ethane, only 3. 0 kcal/mol 6

Types of Strain…. . n Torsional strain: Destabilization due to the repulsion between pairs of bonds caused by the electrostatic repulsion of the electrons in the bonds. Groups are eclipsed. n Steric strain (or steric hindrance): Destabilization due to the repulsion between the electron clouds of atoms or groups. Groups try to occupy some common space. n Angle strain: Destabilisation due to distortion of a bond angle from it's optimum value caused by the electrostatic repulsion of the electrons in the bonds. e. g. cyclopropane 7

Example 2…. . Propane Conformers Note: slight increase in torsional strain due to the more bulky methyl group. 8
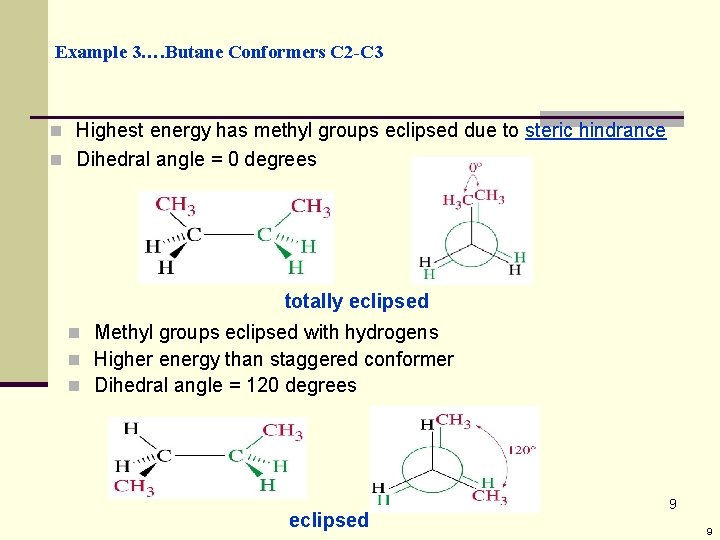
Example 3…. Butane Conformers C 2 -C 3 n Highest energy has methyl groups eclipsed due to steric hindrance n Dihedral angle = 0 degrees totally eclipsed n Methyl groups eclipsed with hydrogens n Higher energy than staggered conformer n Dihedral angle = 120 degrees eclipsed 9 9

Example 3…. Butane Conformers C 2 -C 3 staggered gauche anti n Gauche, staggered conformer n Methyls closer than in anti conformer n Dihedral angle = 60 degrees gauche n Lowest energy has methyl groups anti, staggered conformer. n Dihedral angle = 180 degrees anti 10 10

Example 3…. Butane Conformers C 2 -C 3 11

Conformational Analysis of Higher Alkanes The most stable conformation of unbranched alkanes has anti relationships between carbons 12

Introduction to Cycloalkanes • Besides torsional strain and steric strain, the conformations of cycloalkanes are also affected by angle strain. • Angle strain is an increase in energy when bond angles deviate from the optimum tetrahedral angle of 109. 5°. • The Baeyer strain theory was formulated when it was thought that rings were flat. It states that larger rings would be very highly strained, as their bond angles would be very different from the optimum 109. 5°. The bond angles of a regular polygon with n sides are equal to = 1800 -(3600/n) 13

Note: It turns out that cycloalkanes with more than three C atoms in the ring are not flat molecules. They are puckered to reduce strain. Cyclopropane: reduced overlap of the sp 3 -hybridized orbitals Total strain for cyclopropane = angle strain + torsional strain all adjacent CH 2 groups are eclipsed 14

Cyclobutane: Not Planar; reduced angle and torsional strain relative to cyclopropane Puckering partially relieves torsional strain Cyclopentane: planar conformation is strain free according to Baeyer; however, there is considerable torsional strain (10 H-H eclipsing interactions) Envelope and half-chair conformations relieve much of the torsional strain 15

Cyclohexane: In reality, cyclohexane adopts a puckered “chair” conformation, which is more stable than any possible other conformation. The chair conformation is so stable because it eliminates angle strain (all C—C—C angles are 109. 5°), and torsional strain (all hydrogens on adjacent C atoms are staggered). 16

• In cyclohexane, three C atoms pucker up and three C atoms pucker down, alternating around the ring. • Each C in cyclohexane has two different kinds of hydrogens: (1) axial hydrogens are located above and below the ring (along a perpendicular axis); (2) equatorial hydrogens are located in the plane of the ring (around the equator). 17

18

Ring-flipping interconverts axial and equatorial hydrogens in cyclohexane • An important conformational change in cyclohexane involves “ringflipping. ” Ring-flipping is a two-step process. • As a result of a ring flip, the up carbons become down carbons, and the down carbons become up carbons. • Axial and equatorial H atoms are also interconverted during a ring-flip. Axial H atoms become equatorial H atoms, and equatorial H atoms become axial H atoms. 19

Ring-flipping interconverts axial and equatorial hydrogens in cyclohexane • The chair forms of cyclohexane are 7 kcal/mol more stable than the boat forms. • The boat conformation is destabilized by torsional strain because the hydrogens on the four carbon atoms in the plane are eclipsed. • Additionally, there is steric strain because two hydrogens at either end of the boat, the “flag pole” hydrogens, are forced close to each other. 20

Views of the boat conformation of cyclohexane…. . Newman Projection 21
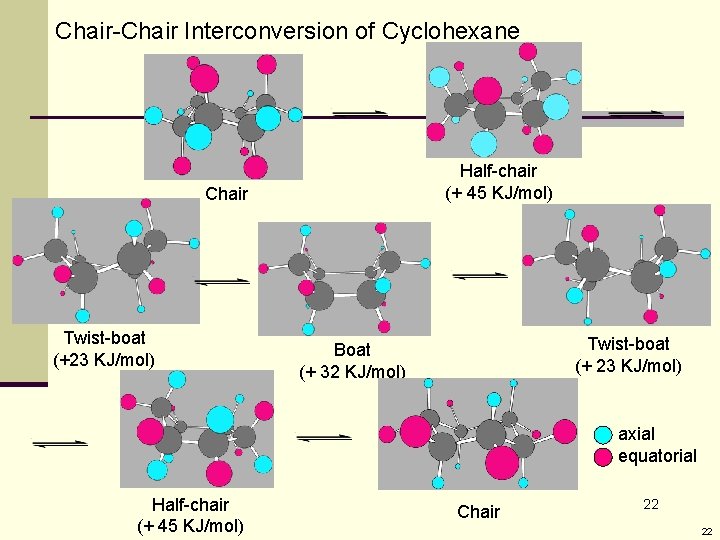
Chair-Chair Interconversion of Cyclohexane Half-chair (+ 45 KJ/mol) Chair Twist-boat (+23 KJ/mol) Twist-boat (+ 23 KJ/mol) Boat (+ 32 KJ/mol) axial equatorial Half-chair (+ 45 KJ/mol) Chair 22 22

Energy Profile for the Chair-Chair Interconversion of Cyclohexane Ring-flip Note: All axial bonds become equatorial 23

Conformations of Monosubstituted Cyclohexanes • Two possible conformations of cyclohexane are different, so they are not equally stable. • Larger axial substituents create unfavorable) 1, 3 -diaxial interactions. 5% destabilizing (and thus 95% More stable 24

Equatorial Conformation is Preferred……. Why? ? Axial Methyl group is Gauche to C 3 in the ring Equatorial Methyl Group is Anti to C 3 in the ring 25
![Note: With a very large substituent like tert-butyl [(CH 3)3 C-], essentially none of Note: With a very large substituent like tert-butyl [(CH 3)3 C-], essentially none of](http://slidetodoc.com/presentation_image/2dfdf604e0cb84e9f17a2efedd16ee73/image-26.jpg)
Note: With a very large substituent like tert-butyl [(CH 3)3 C-], essentially none of the conformation containing an axial tert-butyl group is present at room temperature, so the ring is essentially anchored in a single conformation having an equatorial tert-butyl group. 26

Conformational Analysis of Disubstituted Cyclohexanes • In disubstituted cyclohexanes, the steric effects of both substituents must be taken into account in both conformations • There are two isomers of 1, 2 -dimethylcyclohexane: cis and trans • In the cis isomer, both methyl groups are on the same face of the ring, and compound can exist in two chair conformations • Consider the sum of all interactions; In cis-1, 2, both conformations are equal in energy 3. 8 KJ/mol 15. 2 KJ/mol 27
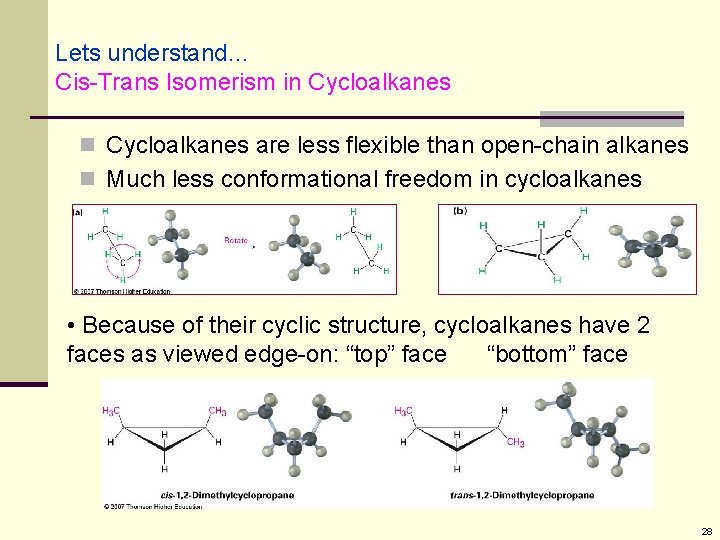
Lets understand… Cis-Trans Isomerism in Cycloalkanes are less flexible than open-chain alkanes n Much less conformational freedom in cycloalkanes • Because of their cyclic structure, cycloalkanes have 2 faces as viewed edge-on: “top” face “bottom” face 28

1, 3 -Dimethylcyclohexane 29

Problems… Q 1. Draw the different possible conformation of 1, 4 Dimethylcyclohexane Q 2. Draw the more stable chair conformer of cis-1 -ethyl 2 -methylcyclohexane Q 3. Draw the more stable conformer of trans-1 -ethyl-2 methylcyclohexane Conformation of dimethyl cyclohexanes Compounds 1, 2 -Dimethyl 1, 3 -Dimethyl 1, 4 -Dimethyl- Cis-isomer a, e or e, a e, e, or a, a a, e or e, a Trans-isomer e, e, or a, a a, e or e, a e, e, or a, a 30

Amine Inversion… • Second kind of conformational isomer • Rapid pyramidal inversion of the amine nitrogen End of (PART A) 31
- Slides: 31