Stereochemistry and conformations Ref Books v Stereochemistry Conformation

Stereochemistry and conformations Ref. Books: v Stereochemistry Conformation & Mechanism - P. S. Kalsi v Organic Chemistry - L. G. Wade v Organic Chemistry - I. L. Finar Vol. 2 v Stereochemistry of Carbon Compounds - E. L. Eliel
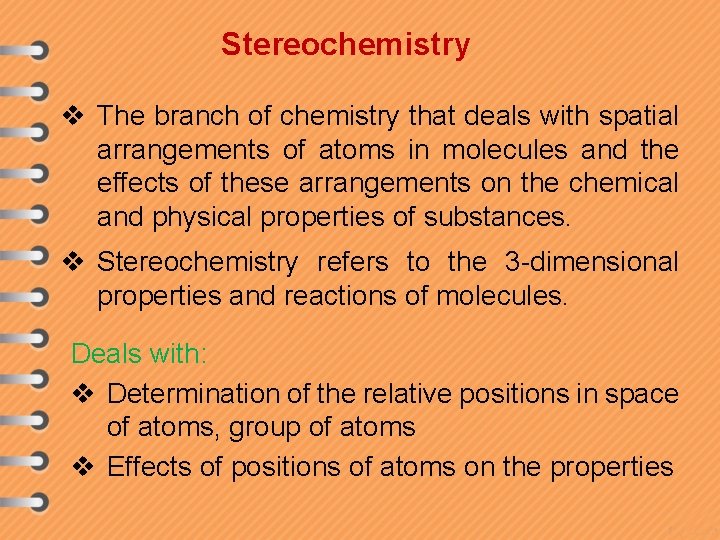
Stereochemistry v The branch of chemistry that deals with spatial arrangements of atoms in molecules and the effects of these arrangements on the chemical and physical properties of substances. v Stereochemistry refers to the 3 -dimensional properties and reactions of molecules. Deals with: v Determination of the relative positions in space of atoms, group of atoms v Effects of positions of atoms on the properties
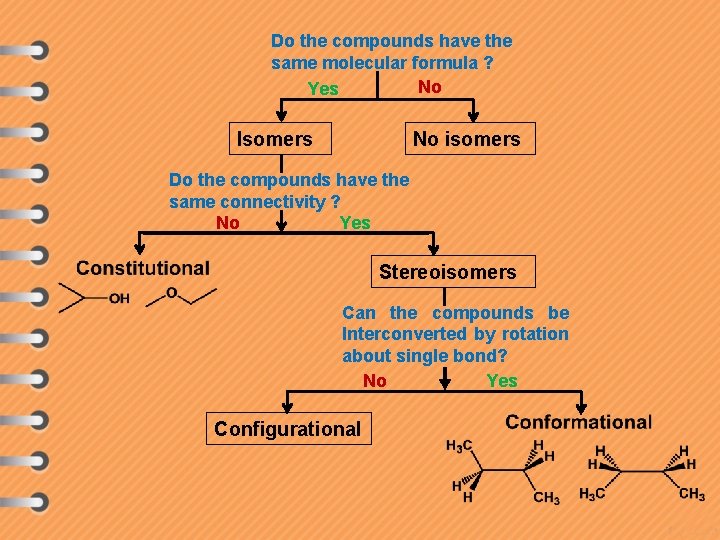
Do the compounds have the same molecular formula ? No Yes Isomers No isomers Do the compounds have the same connectivity ? Yes No Stereoisomers Can the compounds be Interconverted by rotation about single bond? No Yes Configurational
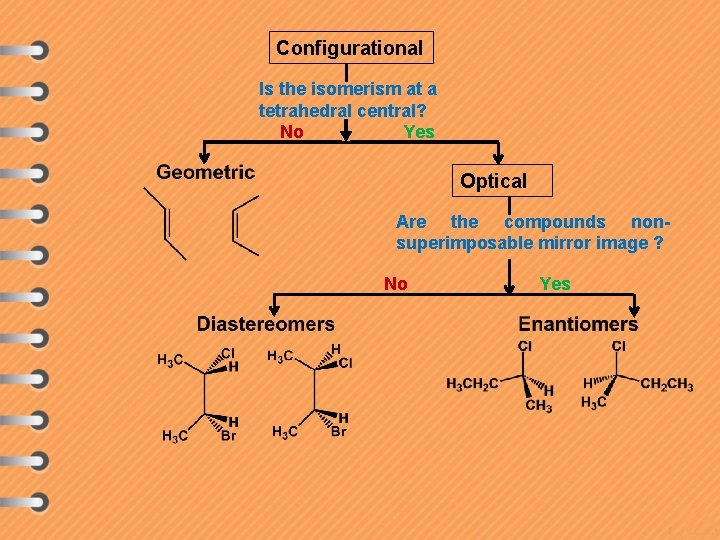
Configurational Is the isomerism at a tetrahedral central? No Yes Optical Are the compounds nonsuperimposable mirror image ? No Yes
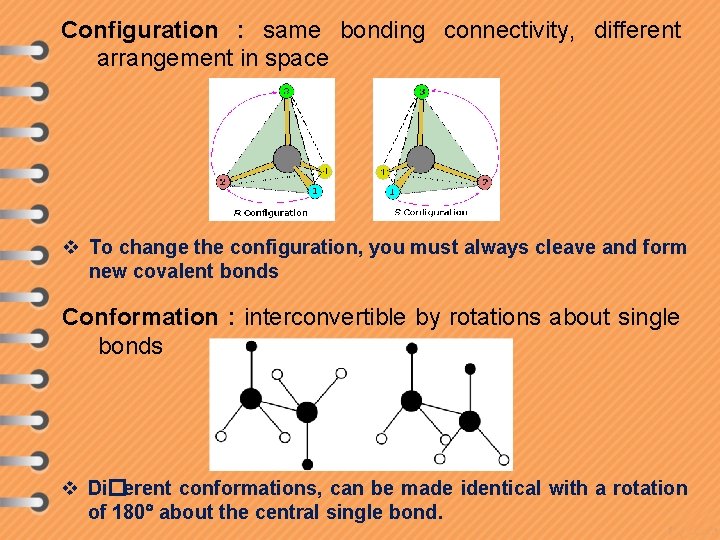
Configuration : same bonding connectivity, different arrangement in space v To change the configuration, you must always cleave and form new covalent bonds Conformation : interconvertible by rotations about single bonds v Di�erent conformations, can be made identical with a rotation of 180 about the central single bond.
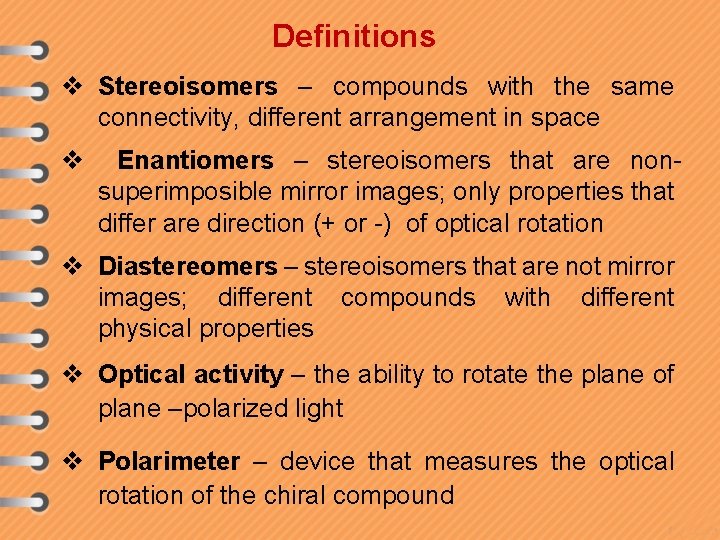
Definitions v Stereoisomers – compounds with the same connectivity, different arrangement in space v Enantiomers – stereoisomers that are nonsuperimposible mirror images; only properties that differ are direction (+ or -) of optical rotation v Diastereomers – stereoisomers that are not mirror images; different compounds with different physical properties v Optical activity – the ability to rotate the plane of plane –polarized light v Polarimeter – device that measures the optical rotation of the chiral compound
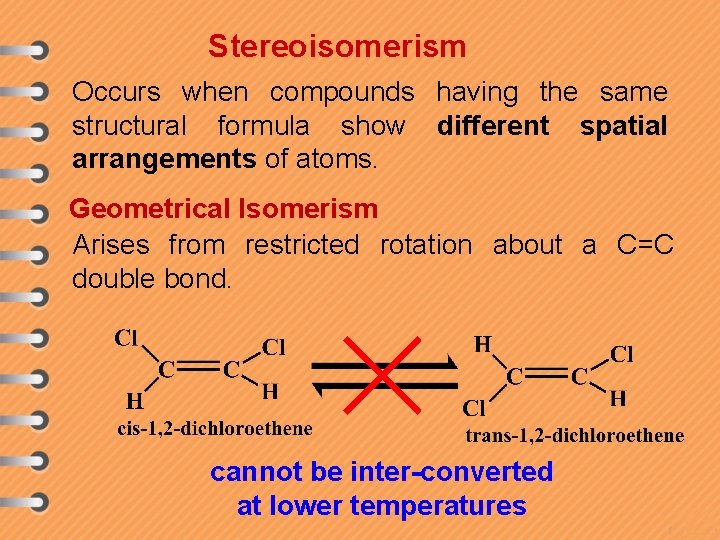
Stereoisomerism Occurs when compounds having the same structural formula show different spatial arrangements of atoms. Geometrical Isomerism Arises from restricted rotation about a C=C double bond. cannot be inter-converted at lower temperatures
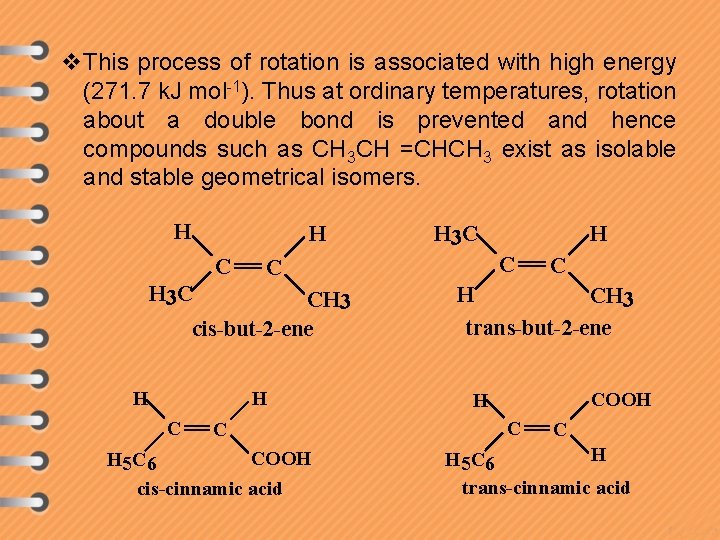
v. This process of rotation is associated with high energy (271. 7 k. J mol-1). Thus at ordinary temperatures, rotation about a double bond is prevented and hence compounds such as CH 3 CH =CHCH 3 exist as isolable and stable geometrical isomers. H H C CH 3 cis-but-2 -ene H C C COOH H 5 C 6 cis-cinnamic acid H C C H 3 C H H 3 C C H CH 3 trans-but-2 -ene COOH H C C H H 5 C 6 trans-cinnamic acid
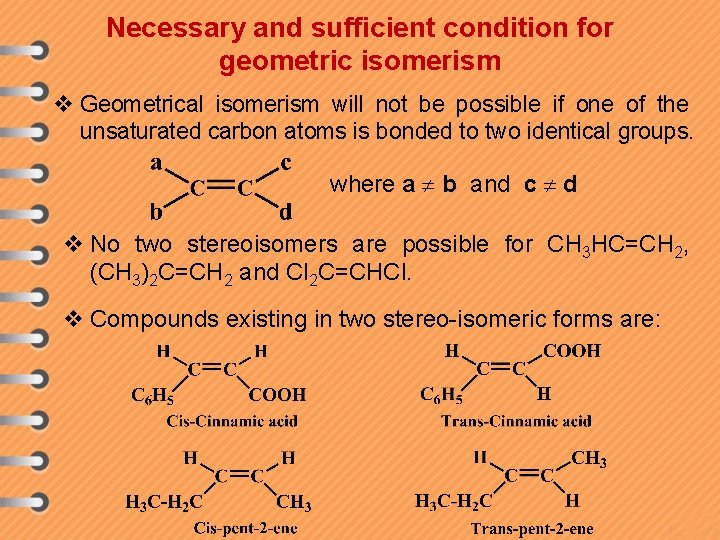
Necessary and sufficient condition for geometric isomerism v Geometrical isomerism will not be possible if one of the unsaturated carbon atoms is bonded to two identical groups. where a b and c d v No two stereoisomers are possible for CH 3 HC=CH 2, (CH 3)2 C=CH 2 and Cl 2 C=CHCl. v Compounds existing in two stereo-isomeric forms are:
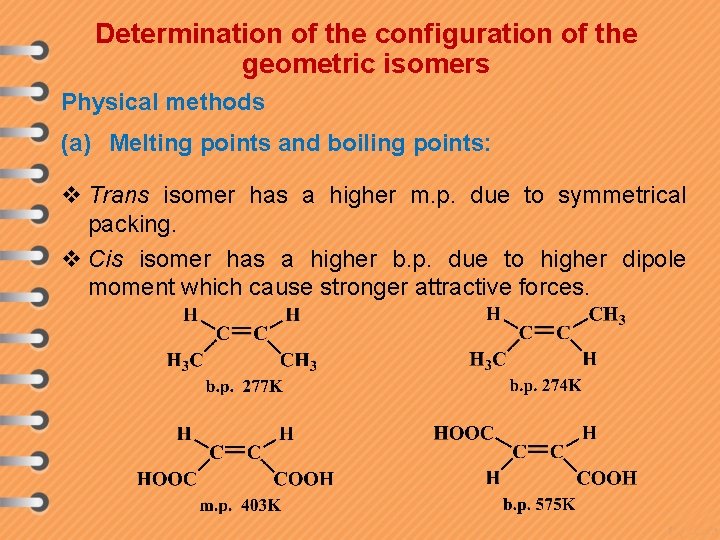
Determination of the configuration of the geometric isomers Physical methods (a) Melting points and boiling points: v Trans isomer has a higher m. p. due to symmetrical packing. v Cis isomer has a higher b. p. due to higher dipole moment which cause stronger attractive forces.

(b) Solubility: Cis-isomers have higher solubilities. Maleic acid 79. 0 g/100 ml at 293 K Fumaric acid 0. 7 g/100 ml at 293 K (c) Dipole moment: In general, cis isomers have the greater dipole moment.
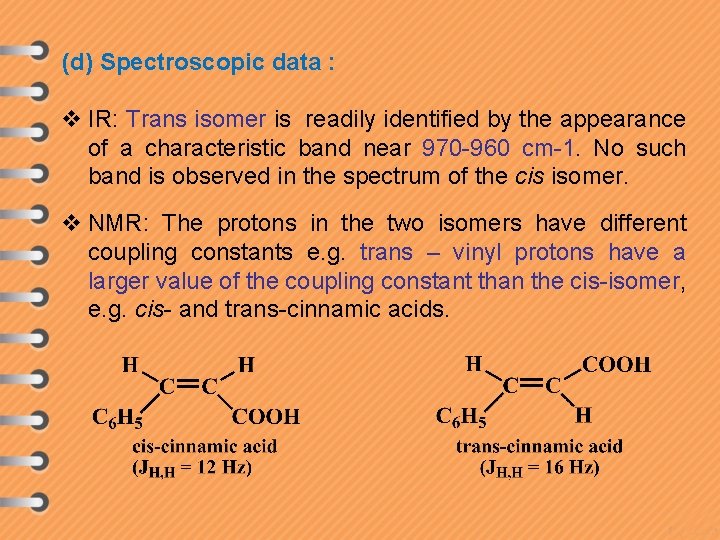
(d) Spectroscopic data : v IR: Trans isomer is readily identified by the appearance of a characteristic band near 970 -960 cm-1. No such band is observed in the spectrum of the cis isomer. v NMR: The protons in the two isomers have different coupling constants e. g. trans – vinyl protons have a larger value of the coupling constant than the cis-isomer, e. g. cis- and trans-cinnamic acids.
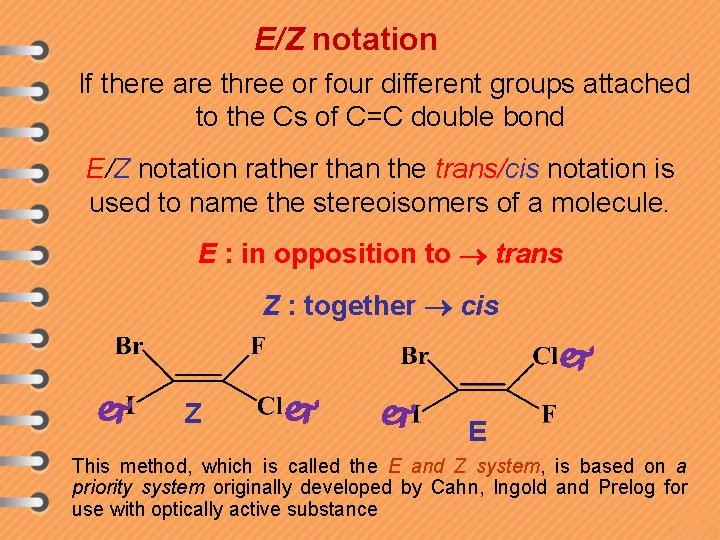
E/Z notation If there are three or four different groups attached to the Cs of C=C double bond E/Z notation rather than the trans/cis notation is used to name the stereoisomers of a molecule. E : in opposition to trans Z : together cis Z E This method, which is called the E and Z system, is based on a priority system originally developed by Cahn, Ingold and Prelog for use with optically active substance
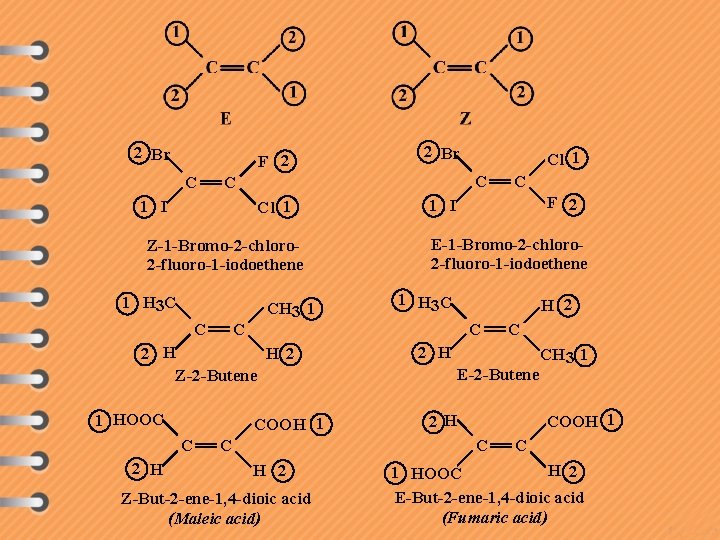
2 Br F 2 C Cl 1 Z-1 -Bromo-2 -chloro 2 -fluoro-1 -iodoethene 1 H 3 C C C CH 3 1 2 H H 2 Z-2 -Butene 1 HOOC 2 H Cl 1 C C 1 I COOH 1 C 2 Br C F 2 1 I E-1 -Bromo-2 -chloro 2 -fluoro-1 -iodoethene 1 H 3 C H 2 C Z-But-2 -ene-1, 4 -dioic acid (Maleic acid) C 2 H E-2 -Butene CH 3 1 COOH 1 2 H C H 2 C C H 2 1 HOOC E-But-2 -ene-1, 4 -dioic acid (Fumaric acid)
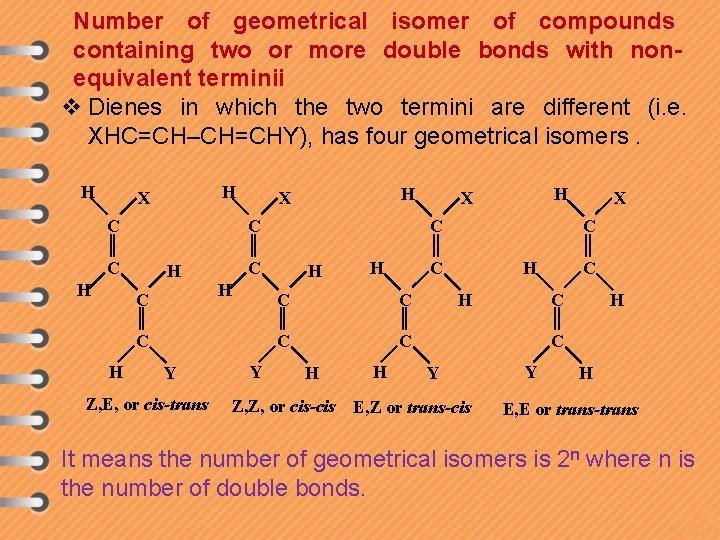
Number of geometrical isomer of compounds containing two or more double bonds with nonequivalent terminii v Dienes in which the two termini are different (i. e. XHC=CH–CH=CHY), has four geometrical isomers. H H X C C C H H C H Y Z, E, or cis-trans Y H X C C C H H X H H C C C X H H C C H C Y Z, Z, or cis-cis E, Z or trans-cis Y H E, E or trans-trans It means the number of geometrical isomers is 2 n where n is the number of double bonds.
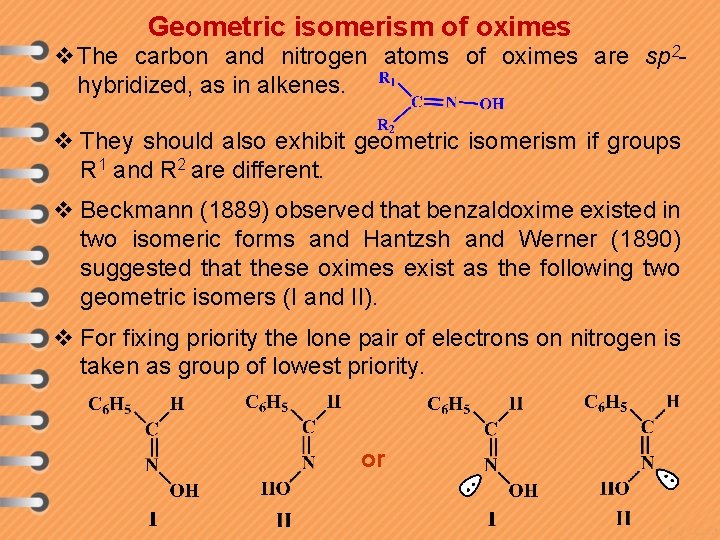
Geometric isomerism of oximes v The carbon and nitrogen atoms of oximes are sp 2 hybridized, as in alkenes. v They should also exhibit geometric isomerism if groups R 1 and R 2 are different. v Beckmann (1889) observed that benzaldoxime existed in two isomeric forms and Hantzsh and Werner (1890) suggested that these oximes exist as the following two geometric isomers (I and II). v For fixing priority the lone pair of electrons on nitrogen is taken as group of lowest priority. or
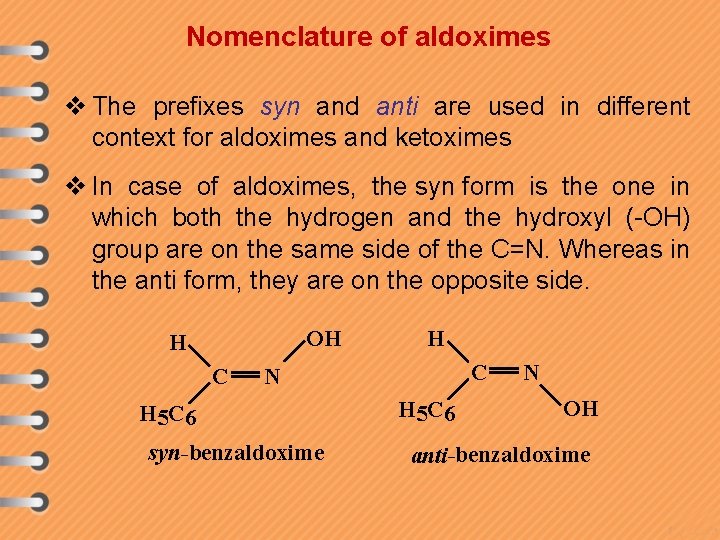
Nomenclature of aldoximes v The prefixes syn and anti are used in different context for aldoximes and ketoximes v In case of aldoximes, the syn form is the one in which both the hydrogen and the hydroxyl (-OH) group are on the same side of the C=N. Whereas in the anti form, they are on the opposite side. OH H C N H 5 C 6 syn-benzaldoxime H 5 C 6 N OH anti-benzaldoxime
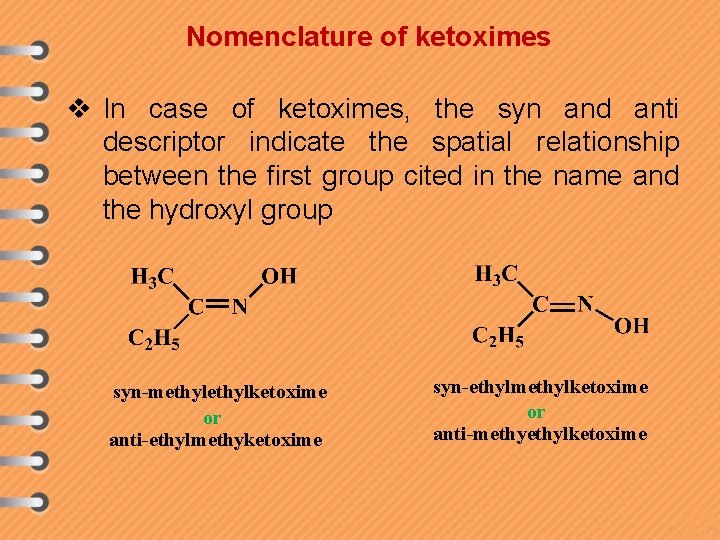
Nomenclature of ketoximes v In case of ketoximes, the syn and anti descriptor indicate the spatial relationship between the first group cited in the name and the hydroxyl group syn-methylketoxime or anti-ethylmethyketoxime syn-ethylmethylketoxime or anti-methylketoxime
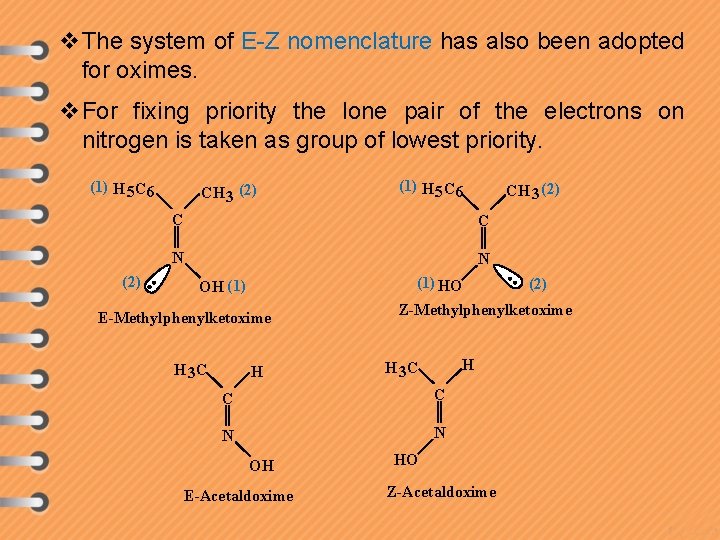
v. The system of E-Z nomenclature has also been adopted for oximes. v. For fixing priority the lone pair of the electrons on nitrogen is taken as group of lowest priority. (1) H 5 C 6 (2) CH 3 (2) (1) H 5 C 6 CH 3 (2) C C N N (1) HO OH (1) E-Methylphenylketoxime H 3 C H Z-Methylphenylketoxime H H 3 C C C N N OH E-Acetaldoxime (2) HO Z-Acetaldoxime
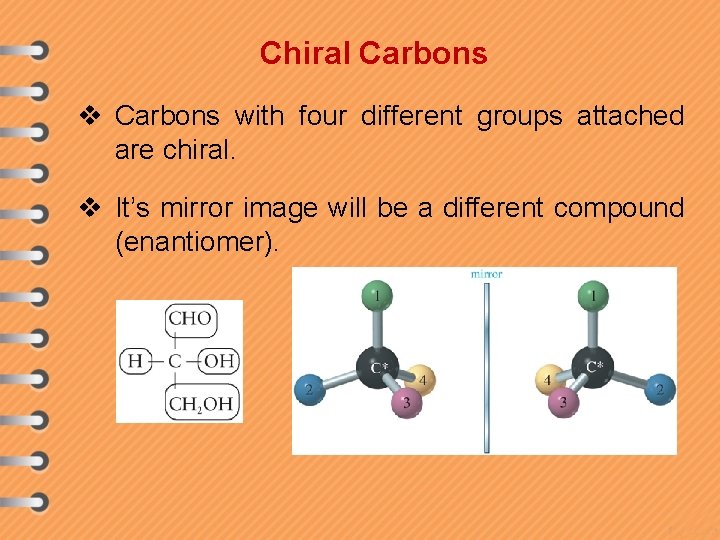
Chiral Carbons v Carbons with four different groups attached are chiral. v It’s mirror image will be a different compound (enantiomer).
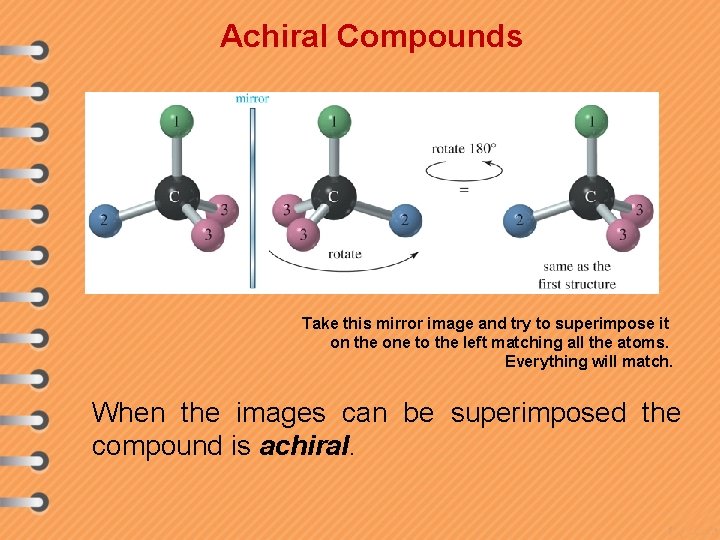
Achiral Compounds Take this mirror image and try to superimpose it on the one to the left matching all the atoms. Everything will match. When the images can be superimposed the compound is achiral.

Chirality - optical activity: discovery v French chemist Louis Pasteur (1848) discovered that crystalline optically inactive sodium ammonium tartarate was a mixture of two types of crystals which were mirror images of each other. v Each type of crystals when dissolved in water was optically active. The specific rotations of the two solutions were exactly equal, but of opposite sign. v In all other properties, the two substances were identical. v As the rotation differs for the two samples in solution in which shapes of crystals disappear, Pasteur proposed that like the two sets of crystals, the molecules making up the crystals were themselves mirror - images of each other and the difference in rotation was due to 'molecular dissymmetry'

Chirality v An object which cannot be superimposed on its mirrorimage is said to be chrial [Greek : Cheir 'Handedness'] and the property of non-superimposability is called chirality. Thus our hands are chiral. v The presence of a chirality centre usually leads to molecular chirality. Such a molecule has no plane of symmetry and exists as a pair of enantiomers. Such a carbon atom is sometimes also referred to as asymmetric carbon atom.
Asymmetric Carbons v The most common feature that leads to chirality in organic compounds is the presence of an asymmetric (or chiral) carbon atom. (A carbon atom that is bonded to four different groups) v In general: v no asymmetric C usually achiral v 1 asymmetric C always chiral v > 2 asymmetric C may or may not be chiral
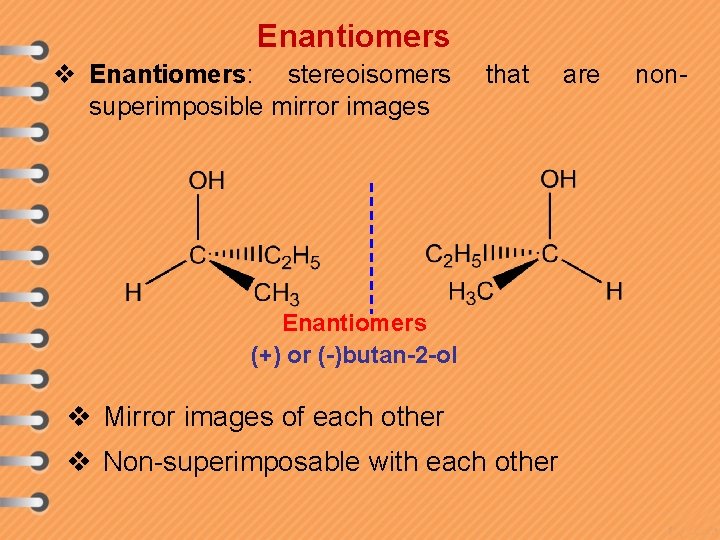
Enantiomers v Enantiomers: stereoisomers superimposible mirror images that Enantiomers (+) or (-)butan-2 -ol v Mirror images of each other v Non-superimposable with each other are non-
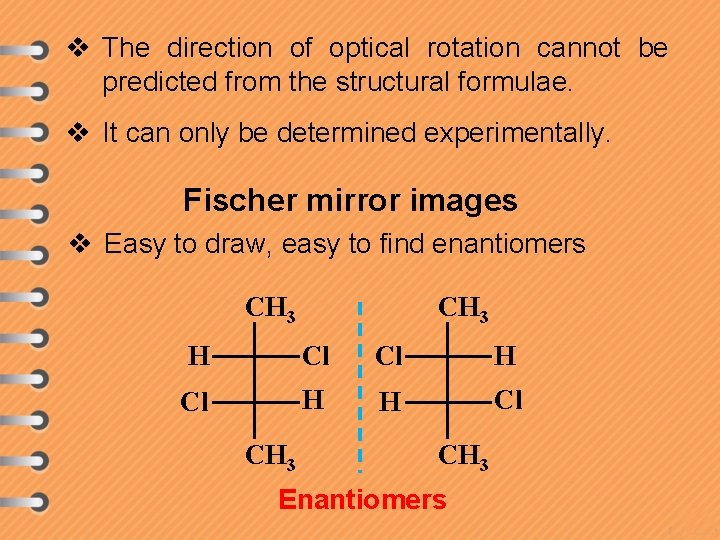
v The direction of optical rotation cannot be predicted from the structural formulae. v It can only be determined experimentally. Fischer mirror images v Easy to draw, easy to find enantiomers CH 3 H Cl Cl H H Cl CH 3 Enantiomers
Properties of enantiomers v Same boiling point, melting point, and density. v Same refractive index. v Rotate the plane of polarized light in the same magnitude, but in opposite directions. v Different interaction with other chiral molecules: v. Active site of enzymes is selective for a specific enantiomer. v. Taste buds and scent receptors are also chiral. Enantiomers may have different smells.
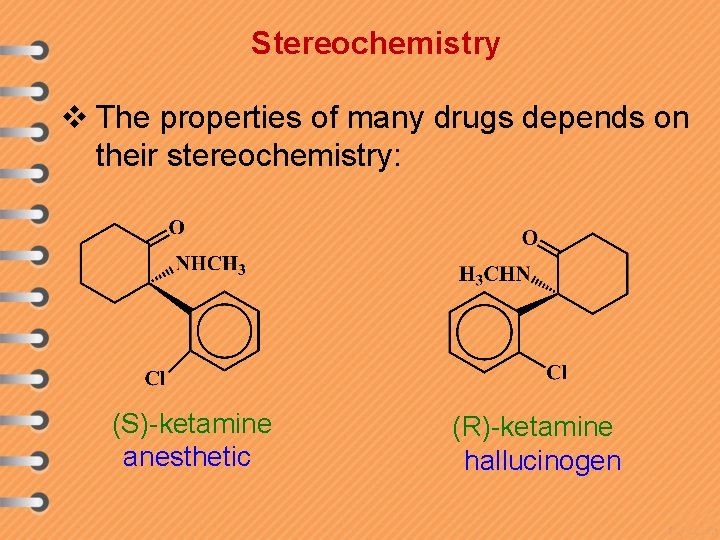
Stereochemistry v The properties of many drugs depends on their stereochemistry: (S)-ketamine anesthetic (R)-ketamine hallucinogen
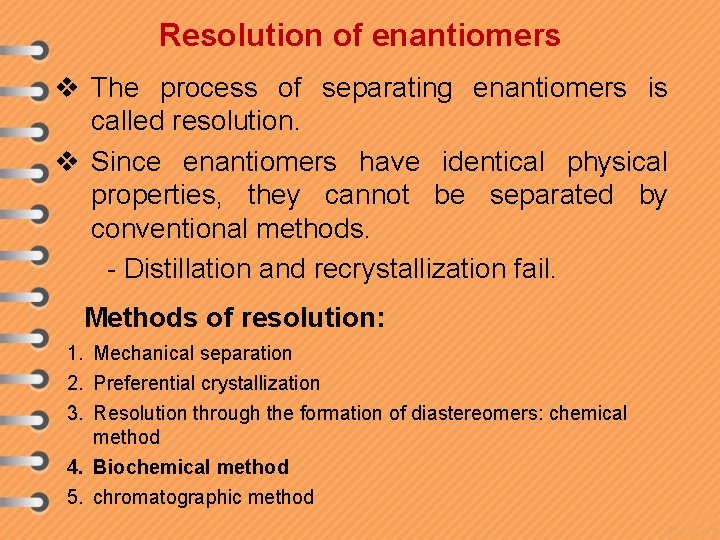
Resolution of enantiomers v The process of separating enantiomers is called resolution. v Since enantiomers have identical physical properties, they cannot be separated by conventional methods. - Distillation and recrystallization fail. Methods of resolution: 1. Mechanical separation 2. Preferential crystallization 3. Resolution through the formation of diastereomers: chemical method 4. Biochemical method 5. chromatographic method
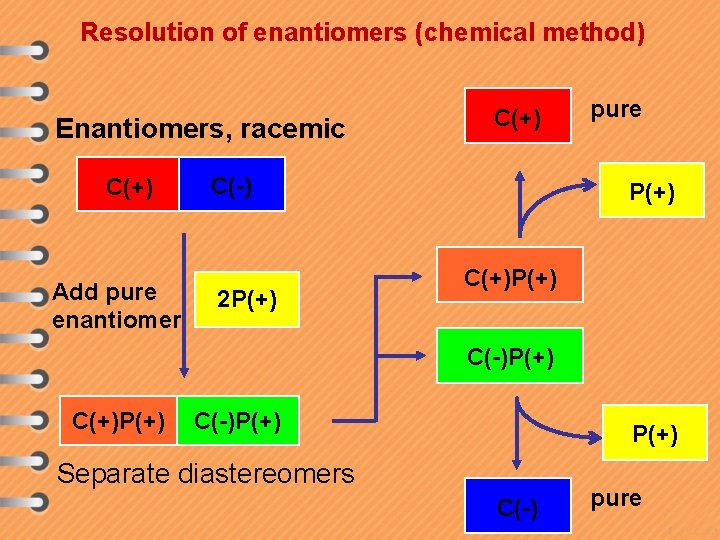
Resolution of enantiomers (chemical method) Enantiomers, racemic C(+) Add pure enantiomer C(+) C(-) 2 P(+) pure P(+) C(+)P(+) C(-)P(+) Separate diastereomers C(-) pure
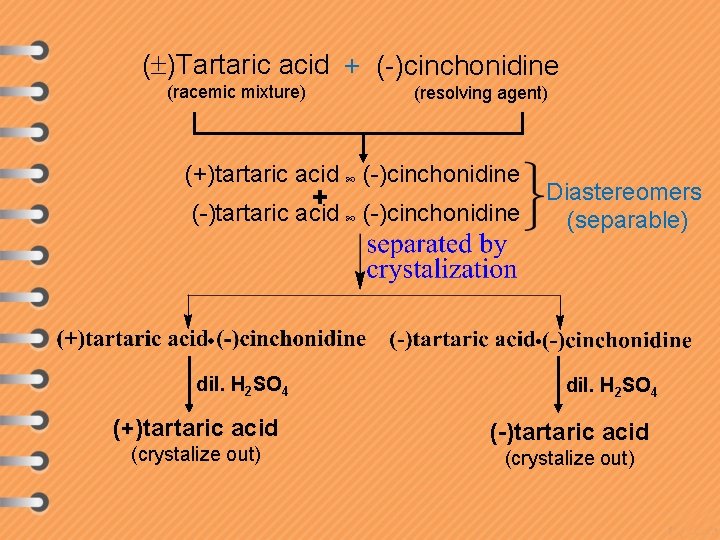
( )Tartaric acid + (-)cinchonidine (racemic mixture) (resolving agent) (+)tartaric acid (-)cinchonidine + (-)tartaric acid (-)cinchonidine dil. H 2 SO 4 (+)tartaric acid (crystalize out) Diastereomers (separable) dil. H 2 SO 4 (-)tartaric acid (crystalize out)
Biochemical Method Microorganisms or enzymes are highly stereoselective. v (+)-Glucose plays an important role in animal metabolism and fermentation, but (-)-glucose is not metabolized by animals, and furthermore cannot be fermented by yeasts. v Penicillium glaucum, consumes only the (+)-enantiomer when fed with a mixture of equal quantities of (+)-and ()-tartaric acids. v Hormonal activity of (-)-adrenaline is many times more than that of its enantiomer. v Limitations: v (i) These reactions are to be carried out in dilute solutions, so isolation of products becomes difficult. v (ii) There is loss of one enantiomer which is consumed by the microorganism. Hence only half of the compound is isolated (partially destructive method).
Chiral biological macromolecules v Proteins § § § Enzimes Structural elements of membrances Receptors v Carbohydrates v Nucleic acids v Chiral ‘building blocks’ of L-amino acids and D-carbohydrates
Biological discrimination of enantiomers v Enantiomers can be distinguished through the use of chiral probes. A polarimeter is one example of a chiral probe. v Enzymes are a type of chiral probe that are found in living systems. v In general, enantiomers do not interact identical with other chiral molecules v Enzymes are chiral, and are capable of distinguishing between enantiomers v Either one has no effect or has a totally different v Usually, only one enantiomers of a pair fits properly into the active site of an enzyme
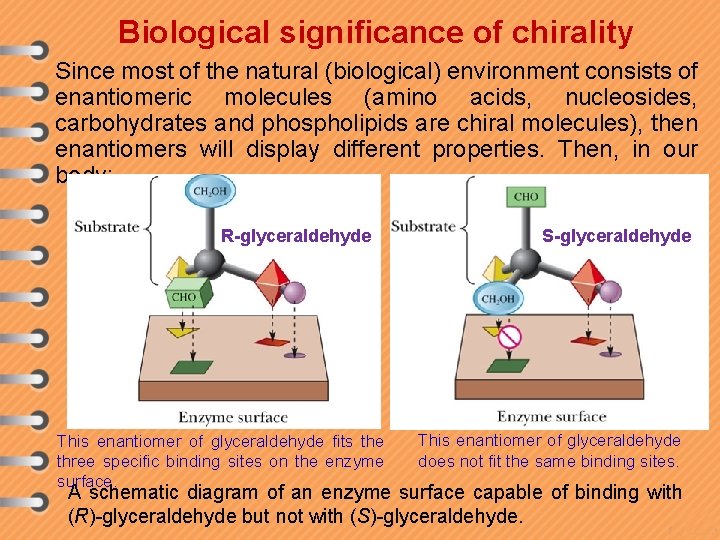
Biological significance of chirality Since most of the natural (biological) environment consists of enantiomeric molecules (amino acids, nucleosides, carbohydrates and phospholipids are chiral molecules), then enantiomers will display different properties. Then, in our body: R-glyceraldehyde This enantiomer of glyceraldehyde fits the three specific binding sites on the enzyme surface. S-glyceraldehyde This enantiomer of glyceraldehyde does not fit the same binding sites. A schematic diagram of an enzyme surface capable of binding with (R)-glyceraldehyde but not with (S)-glyceraldehyde.
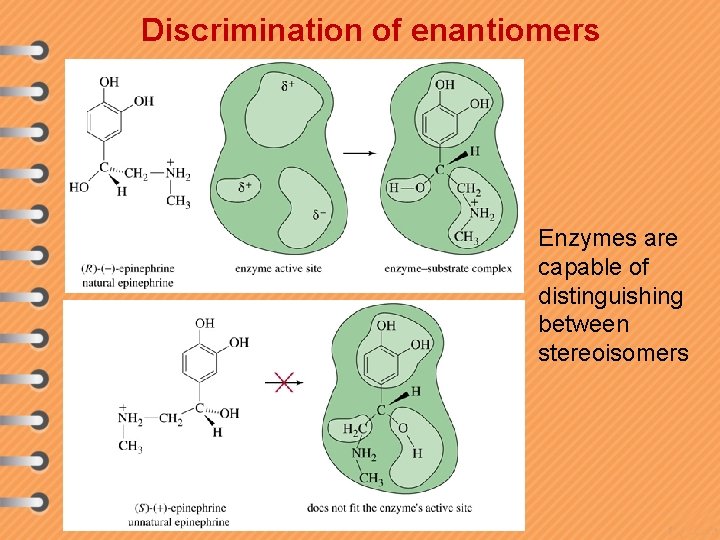
Discrimination of enantiomers Enzymes are capable of distinguishing between stereoisomers
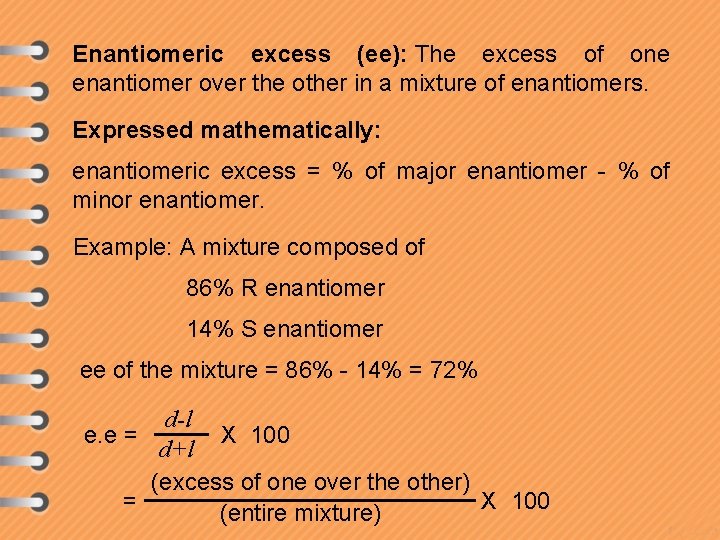
Enantiomeric excess (ee): The excess of one enantiomer over the other in a mixture of enantiomers. Expressed mathematically: enantiomeric excess = % of major enantiomer - % of minor enantiomer. Example: A mixture composed of 86% R enantiomer 14% S enantiomer ee of the mixture = 86% - 14% = 72% d-l e. e = X 100 d+l (excess of one over the other) = X 100 (entire mixture)
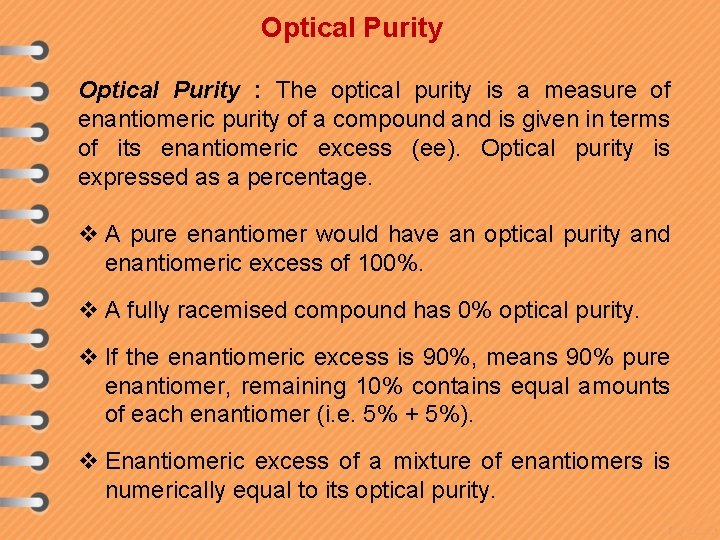
Optical Purity : The optical purity is a measure of enantiomeric purity of a compound and is given in terms of its enantiomeric excess (ee). Optical purity is expressed as a percentage. v A pure enantiomer would have an optical purity and enantiomeric excess of 100%. v A fully racemised compound has 0% optical purity. v If the enantiomeric excess is 90%, means 90% pure enantiomer, remaining 10% contains equal amounts of each enantiomer (i. e. 5% + 5%). v Enantiomeric excess of a mixture of enantiomers is numerically equal to its optical purity.
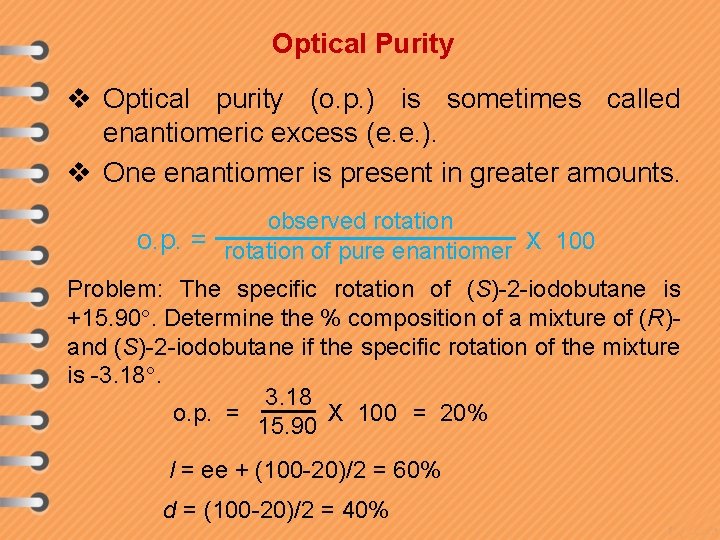
Optical Purity v Optical purity (o. p. ) is sometimes called enantiomeric excess (e. e. ). v One enantiomer is present in greater amounts. observed rotation o. p. = rotation of pure enantiomer X 100 Problem: The specific rotation of (S)-2 -iodobutane is +15. 90. Determine the % composition of a mixture of (R)and (S)-2 -iodobutane if the specific rotation of the mixture is -3. 18 o. p. = X 100 = 20% 15. 90 l = ee + (100 -20)/2 = 60% d = (100 -20)/2 = 40%
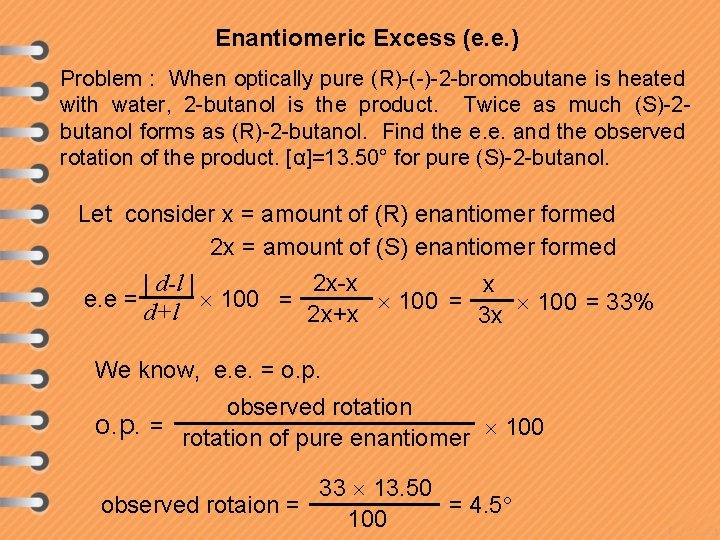
Enantiomeric Excess (e. e. ) Problem : When optically pure (R)-(-)-2 -bromobutane is heated with water, 2 -butanol is the product. Twice as much (S)-2 butanol forms as (R)-2 -butanol. Find the e. e. and the observed rotation of the product. [α]=13. 50° for pure (S)-2 -butanol. Let consider x = amount of (R) enantiomer formed 2 x = amount of (S) enantiomer formed | d-l | 2 x-x x e. e = 100 = 33% d+l 2 x+x 3 x We know, e. e. = o. p. observed rotation o. p. = rotation of pure enantiomer 100 33 13. 50 = 4. 5 observed rotaion = 100
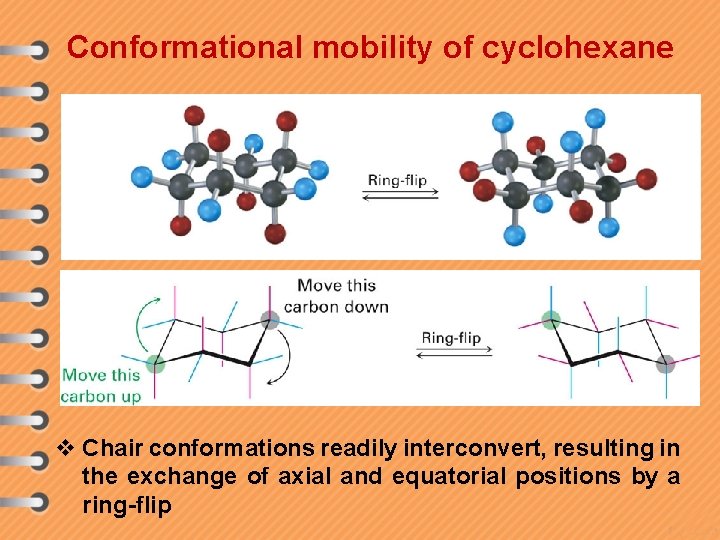
Conformational mobility of cyclohexane v Chair conformations readily interconvert, resulting in the exchange of axial and equatorial positions by a ring-flip
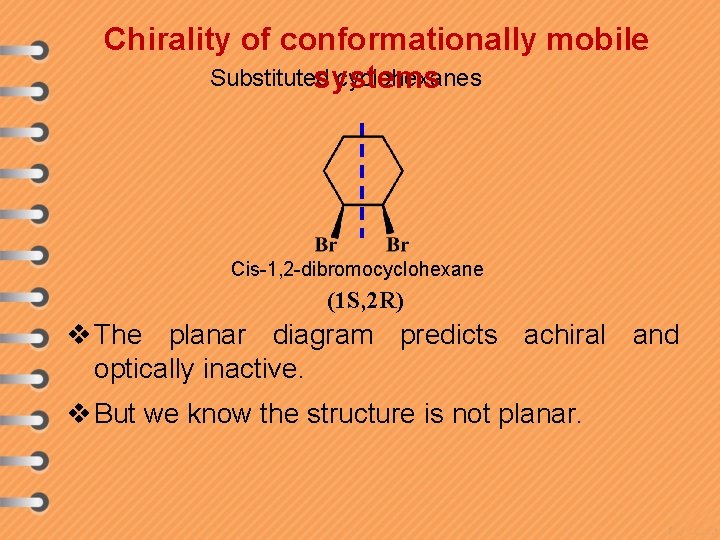
Chirality of conformationally mobile Substituted cyclohexanes systems Cis-1, 2 -dibromocyclohexane (1 S, 2 R) v The planar diagram predicts achiral and optically inactive. v But we know the structure is not planar.
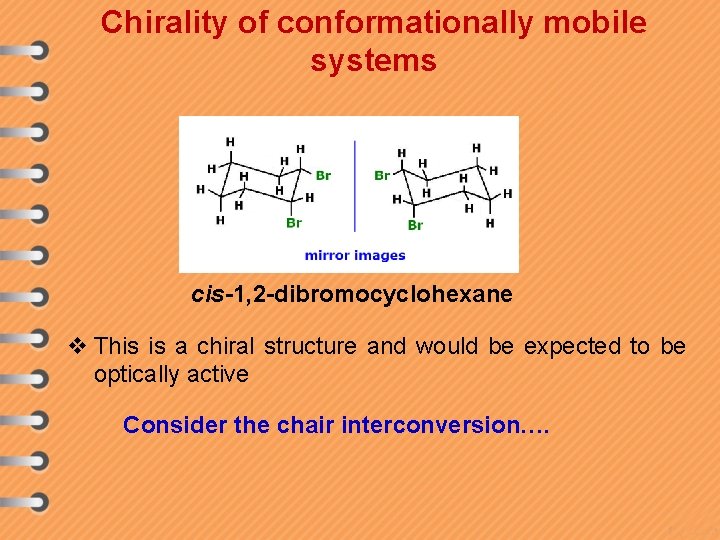
Chirality of conformationally mobile systems cis-1, 2 -dibromocyclohexane v This is a chiral structure and would be expected to be optically active Consider the chair interconversion….
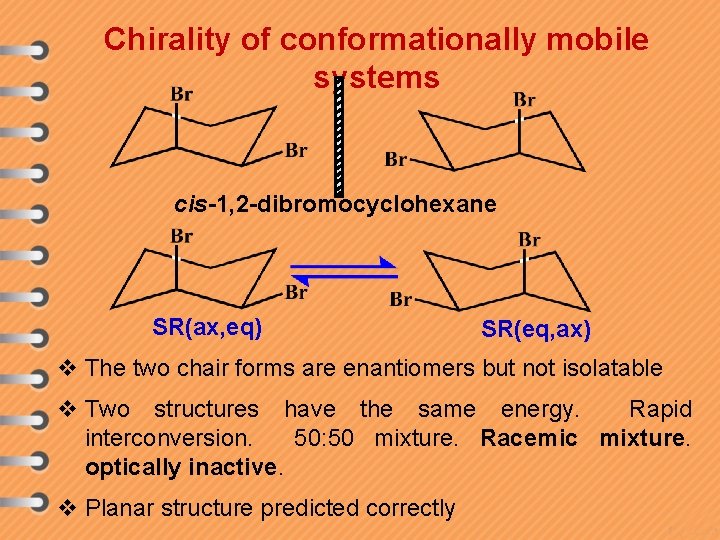
Chirality of conformationally mobile systems cis-1, 2 -dibromocyclohexane SR(ax, eq) SR(eq, ax) v The two chair forms are enantiomers but not isolatable v Two structures have the same energy. Rapid interconversion. 50: 50 mixture. Racemic mixture. optically inactive. v Planar structure predicted correctly
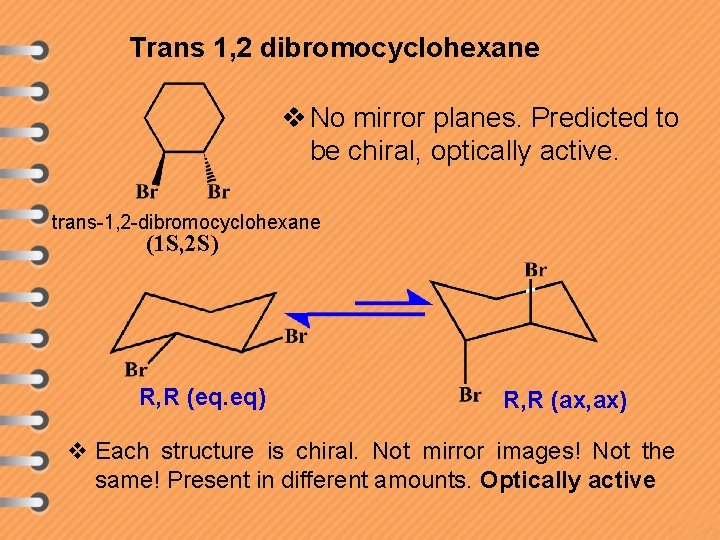
Trans 1, 2 dibromocyclohexane v No mirror planes. Predicted to be chiral, optically active. trans-1, 2 -dibromocyclohexane (1 S, 2 S) R, R (eq. eq) R, R (ax, ax) v Each structure is chiral. Not mirror images! Not the same! Present in different amounts. Optically active

Mobile conformers v If equilibrium exists between two chiral conformers, the molecule is not chiral. v Judge chirality by looking at the most symmetrical conformer. v Cyclohexane can be considered to be planar, on average.
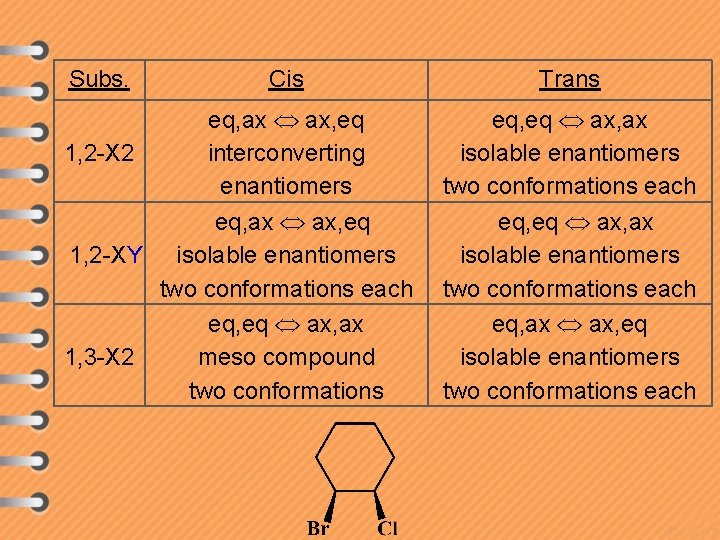
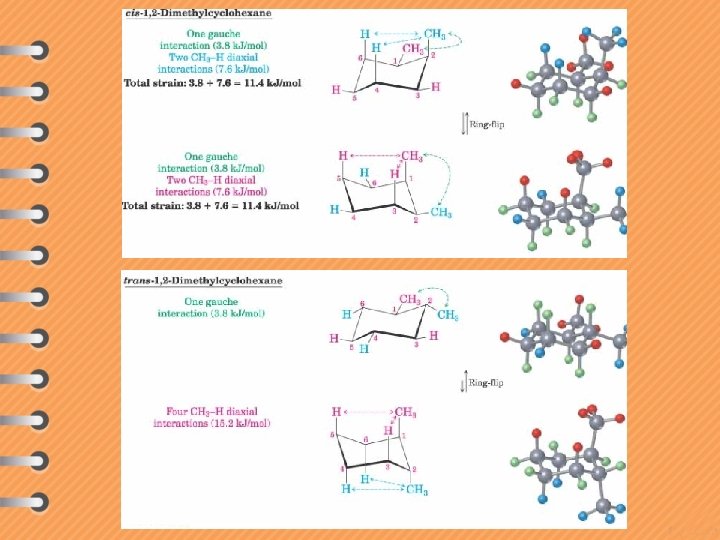
Subs. Cis eq, ax ax, eq 1, 2 -X 2 interconverting enantiomers eq, ax ax, eq 1, 2 -XY isolable enantiomers two conformations each eq, eq ax, ax 1, 3 -X 2 meso compound two conformations Trans eq, eq ax, ax isolable enantiomers two conformations each eq, ax ax, eq isolable enantiomers two conformations each
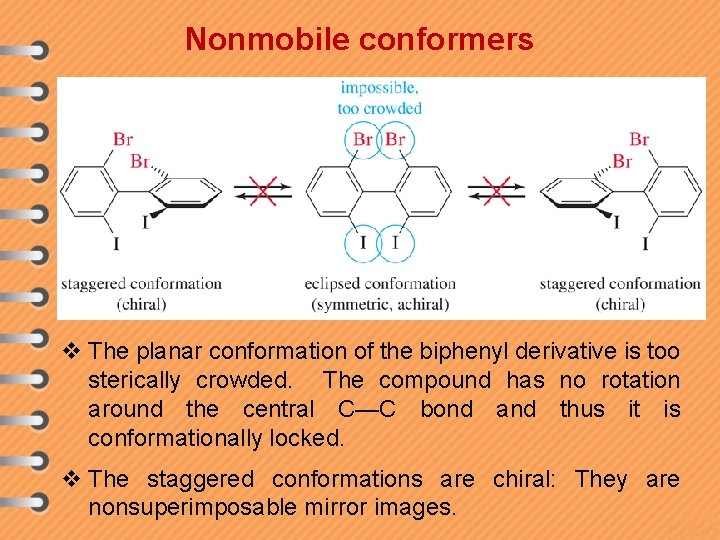
Nonmobile conformers v The planar conformation of the biphenyl derivative is too sterically crowded. The compound has no rotation around the central C—C bond and thus it is conformationally locked. v The staggered conformations are chiral: They are nonsuperimposable mirror images.
Conformational Analysis v The different spatial arrangements that a molecule can adopt due to rotation about σ bonds are called conformations and hence conformational isomers or conformers. v The study of the energy changes that occur during these rotations is called conformational analysis.
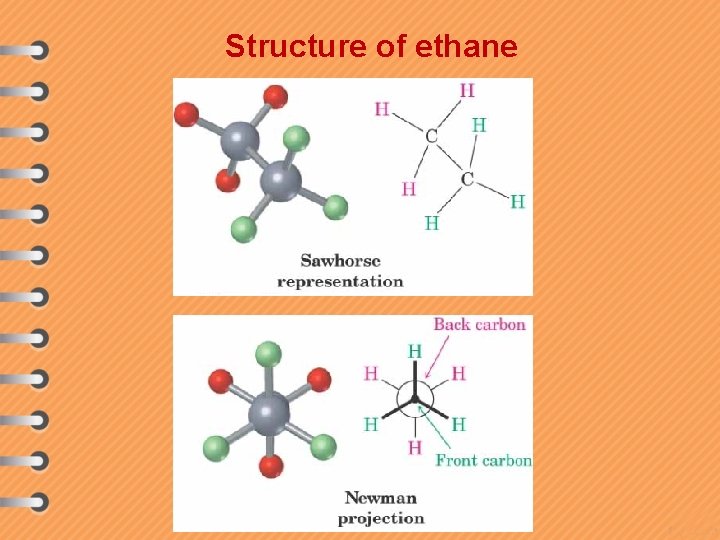
Structure of ethane
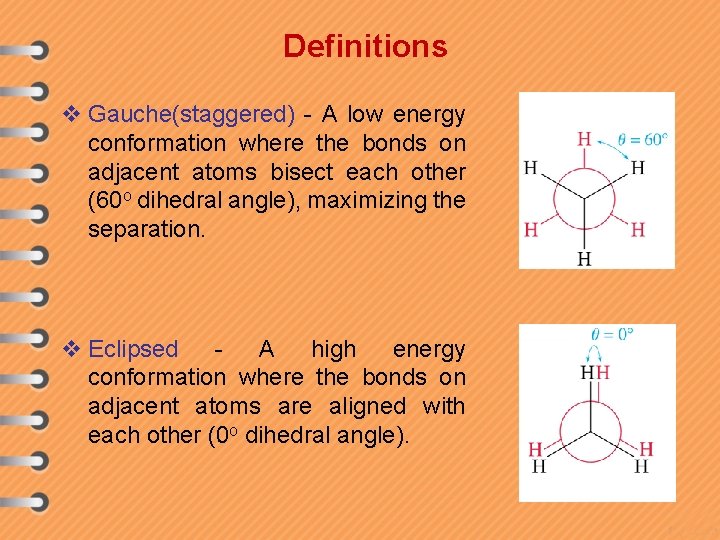
Definitions v Gauche(staggered) - A low energy conformation where the bonds on adjacent atoms bisect each other (60 o dihedral angle), maximizing the separation. v Eclipsed - A high energy conformation where the bonds on adjacent atoms are aligned with each other (0 o dihedral angle).
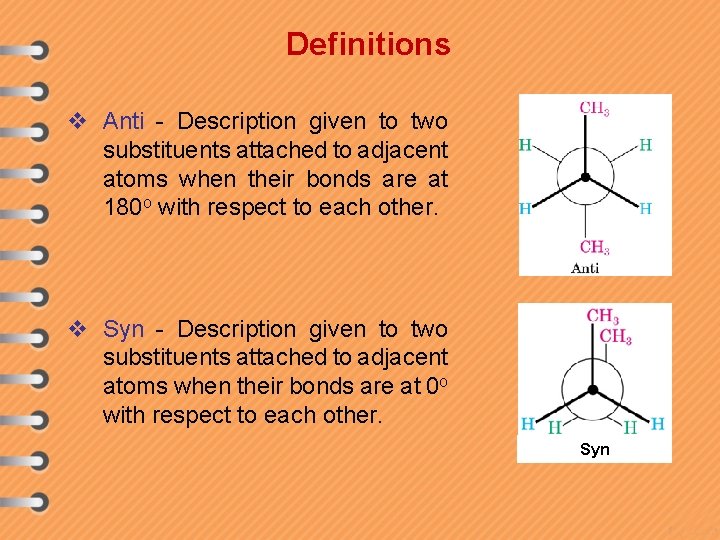
Definitions v Anti - Description given to two substituents attached to adjacent atoms when their bonds are at 180 o with respect to each other. v Syn - Description given to two substituents attached to adjacent atoms when their bonds are at 0 o with respect to each other. Syn
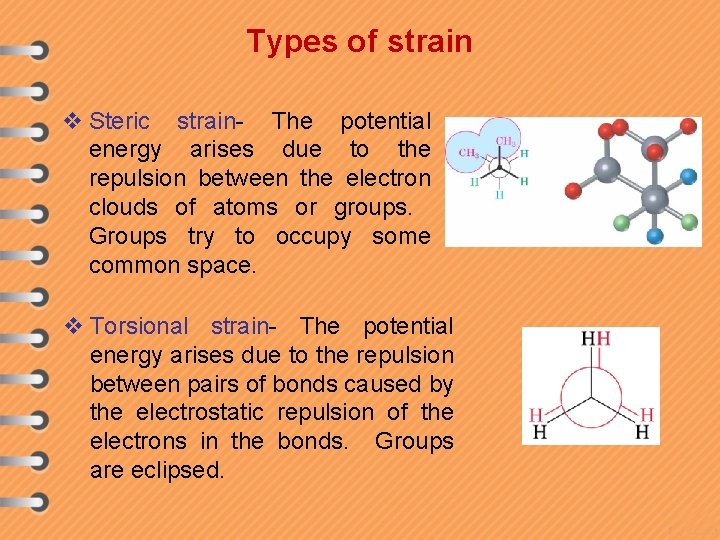
Types of strain v Steric strain- The potential energy arises due to the repulsion between the electron clouds of atoms or groups. Groups try to occupy some common space. v Torsional strain- The potential energy arises due to the repulsion between pairs of bonds caused by the electrostatic repulsion of the electrons in the bonds. Groups are eclipsed.
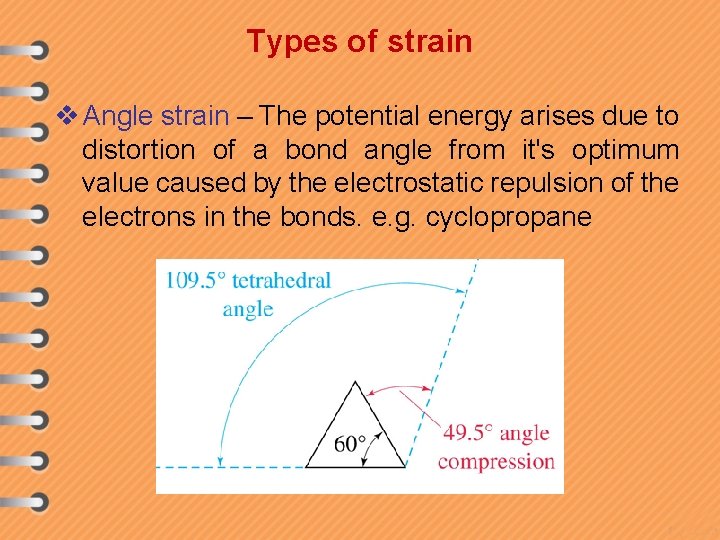
Types of strain v Angle strain – The potential energy arises due to distortion of a bond angle from it's optimum value caused by the electrostatic repulsion of the electrons in the bonds. e. g. cyclopropane
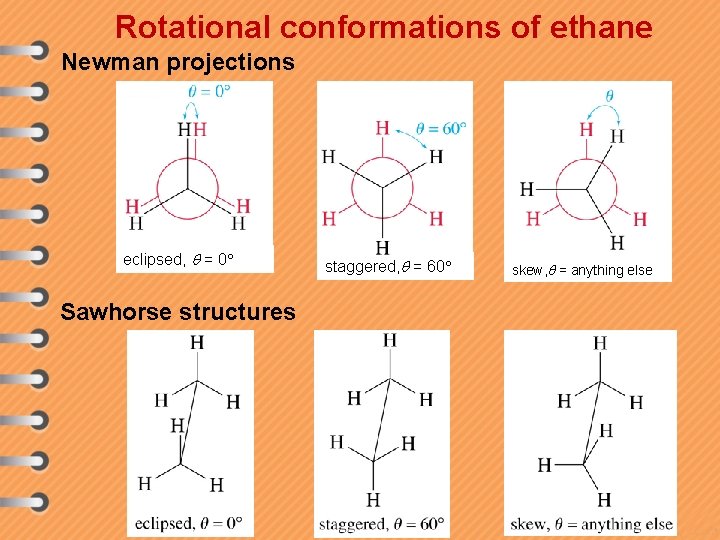
Rotational conformations of ethane Newman projections eclipsed, = 0 Sawhorse structures staggered, = 60 skew, = anything else
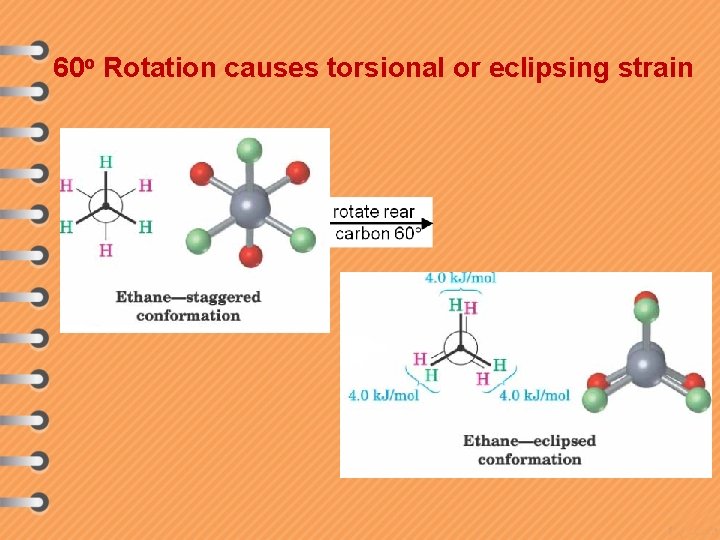
60 o Rotation causes torsional or eclipsing strain
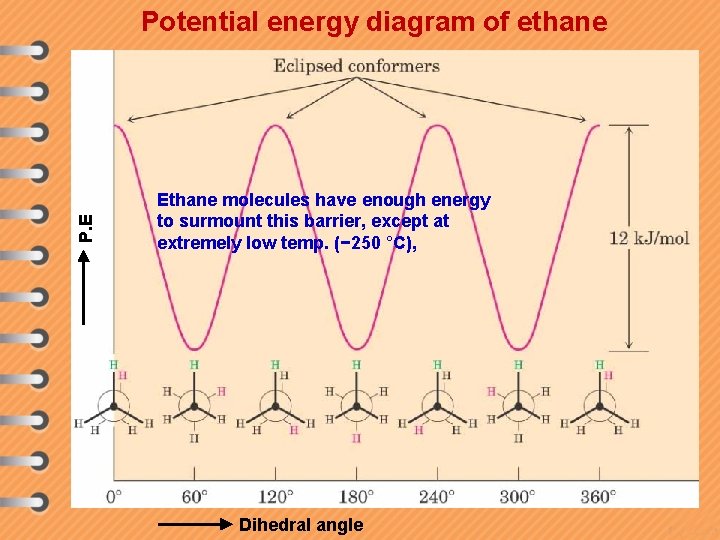
P. E Potential energy diagram of ethane Ethane molecules have enough energy to surmount this barrier, except at extremely low temp. (− 250 °C), Dihedral angle
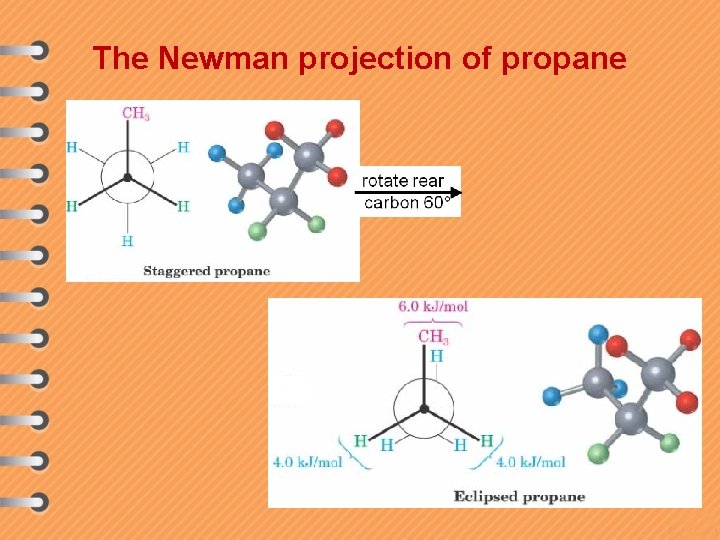
The Newman projection of propane
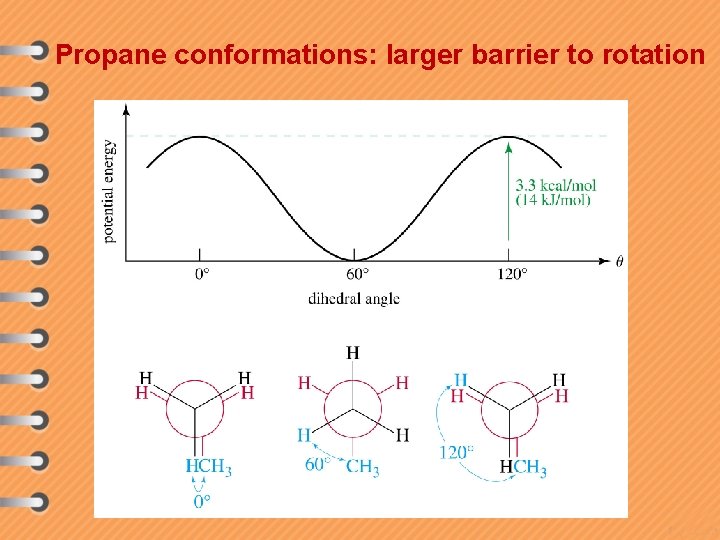
Propane conformations: larger barrier to rotation
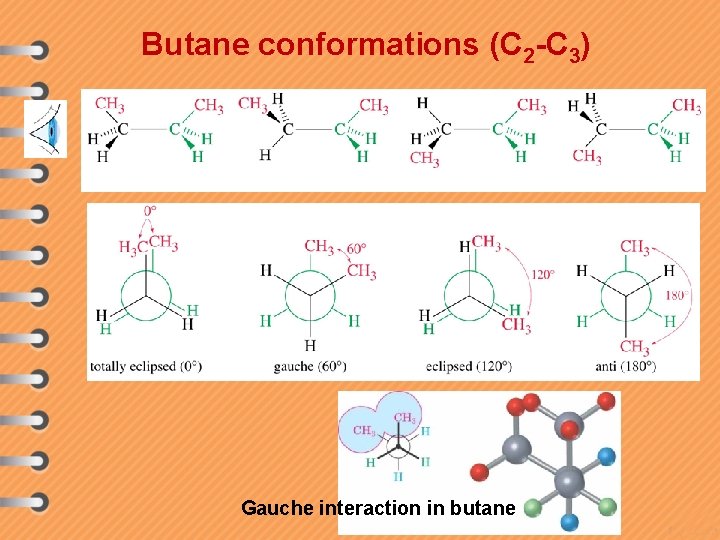
Butane conformations (C 2 -C 3) Gauche interaction in butane
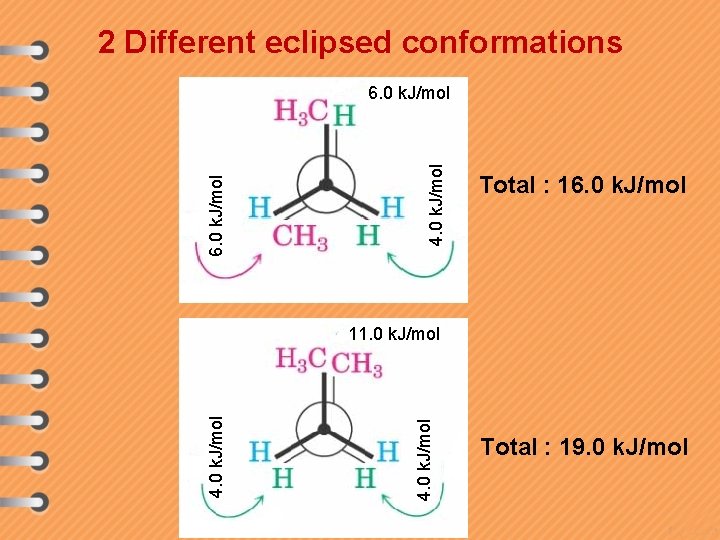
2 Different eclipsed conformations 4. 0 k. J/mol 6. 0 k. J/mol Total : 16. 0 k. J/mol 4. 0 k. J/mol 4. 0 k. J/mol 11. 0 k. J/mol Total : 19. 0 k. J/mol
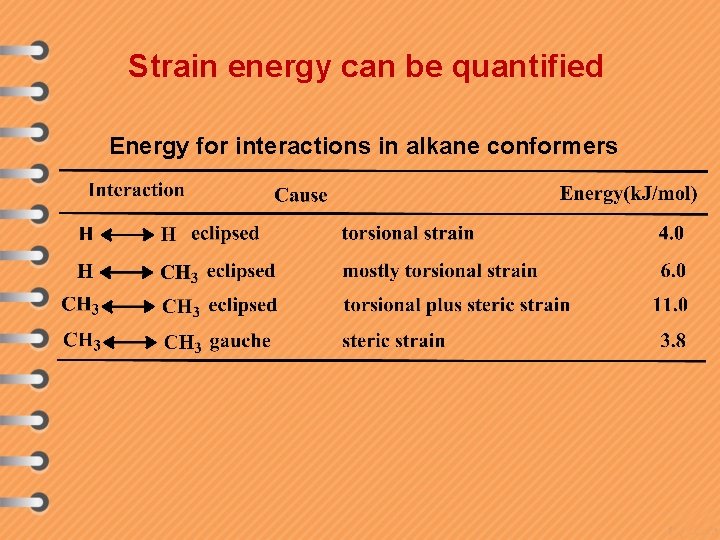
Strain energy can be quantified Energy for interactions in alkane conformers

Potential energy diagram of butane Dihedral angle between methyl groups
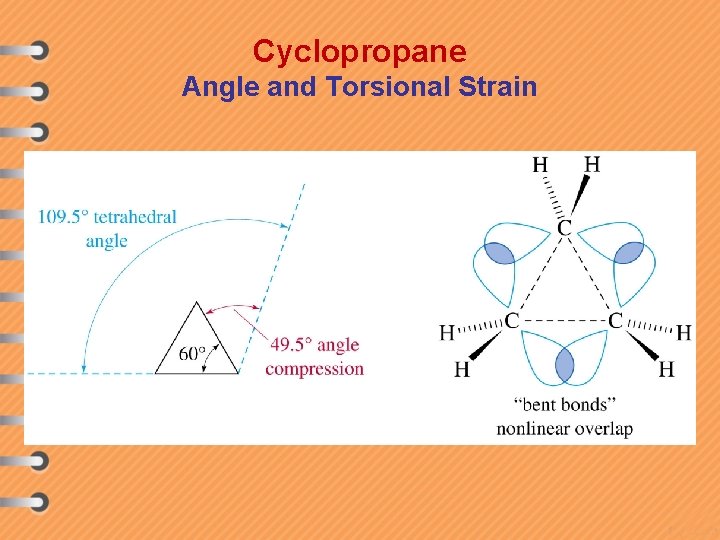
Cyclopropane Angle and Torsional Strain
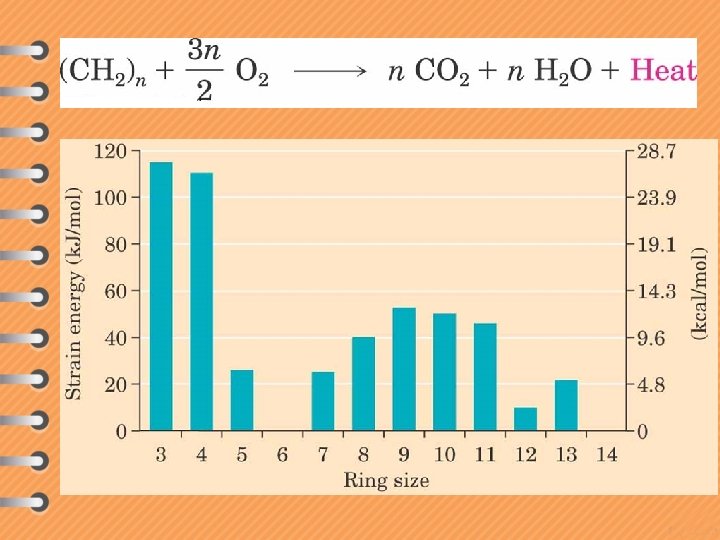
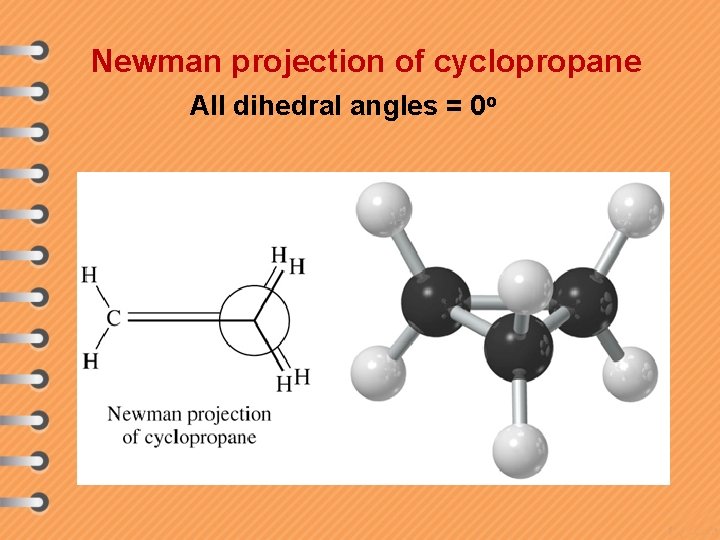
Newman projection of cyclopropane All dihedral angles = 0 o
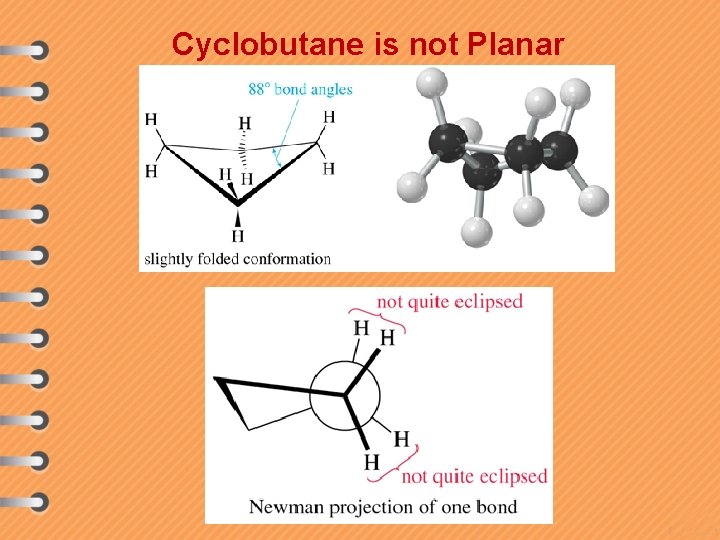
Cyclobutane is not Planar
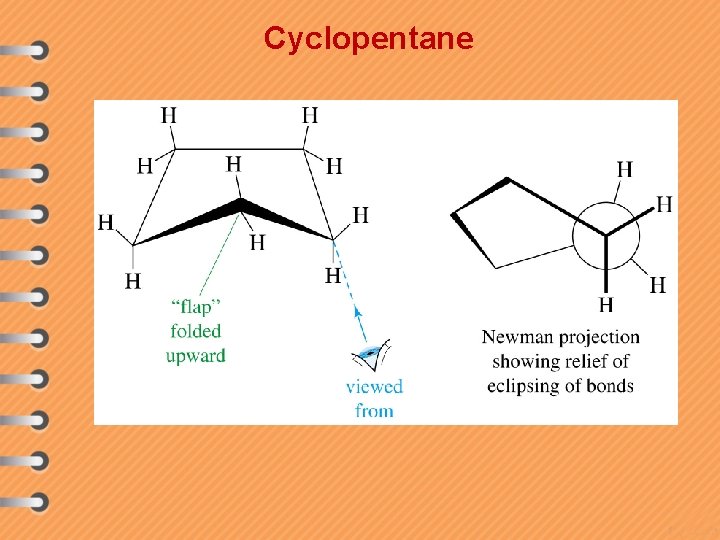
Cyclopentane
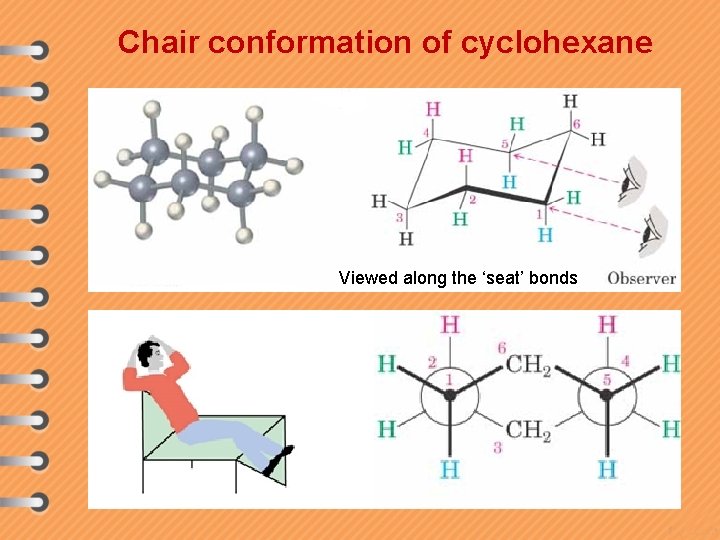
Chair conformation of cyclohexane Viewed along the ‘seat’ bonds
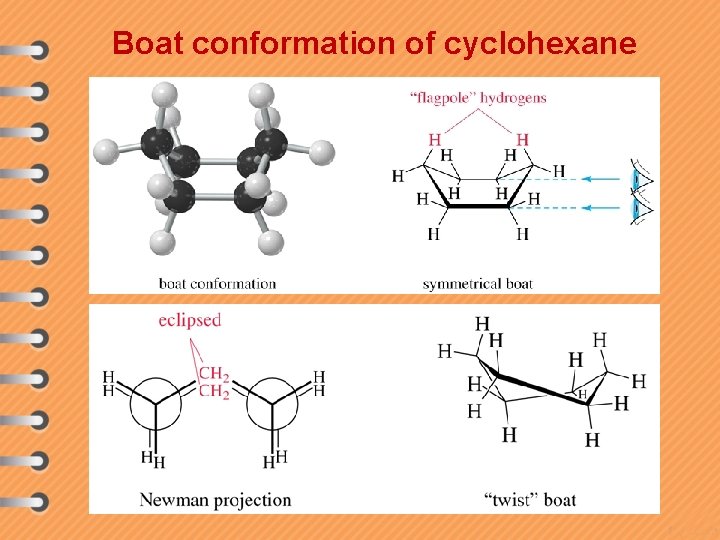
Boat conformation of cyclohexane
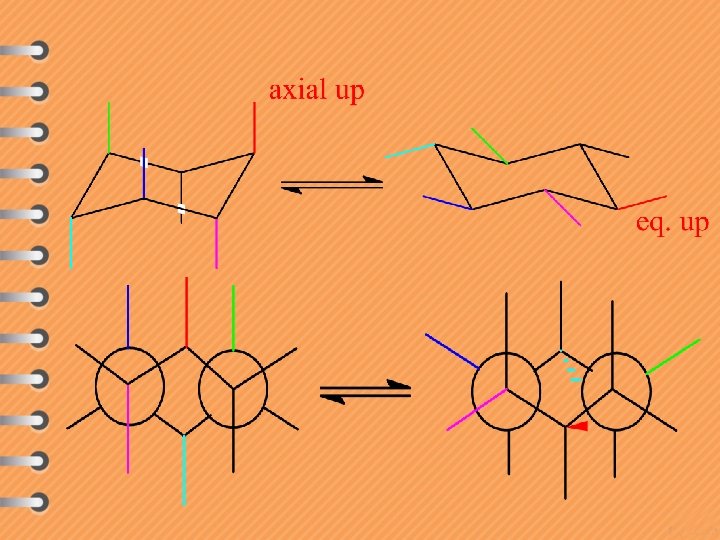
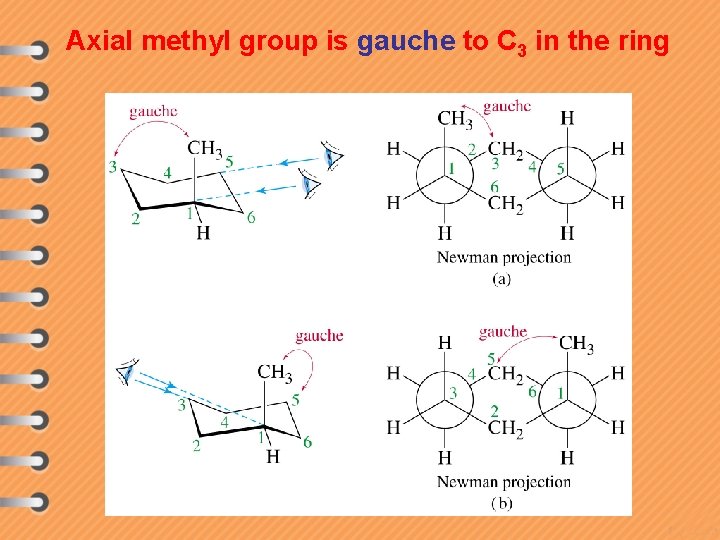
Axial methyl group is gauche to C 3 in the ring
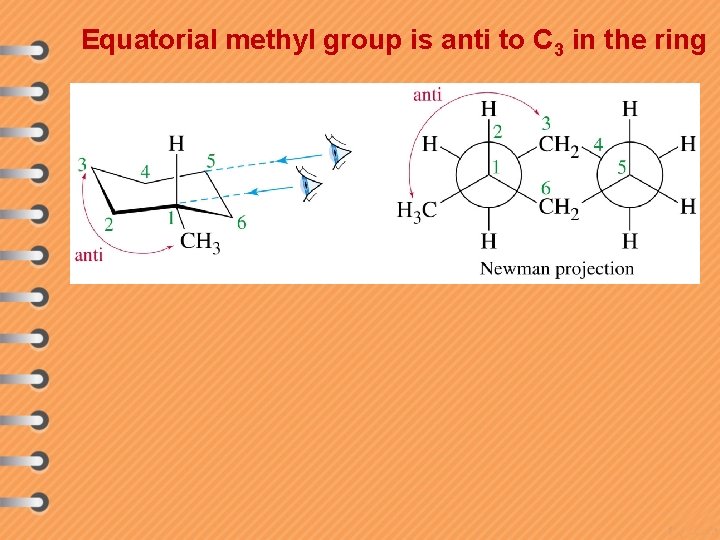
Equatorial methyl group is anti to C 3 in the ring
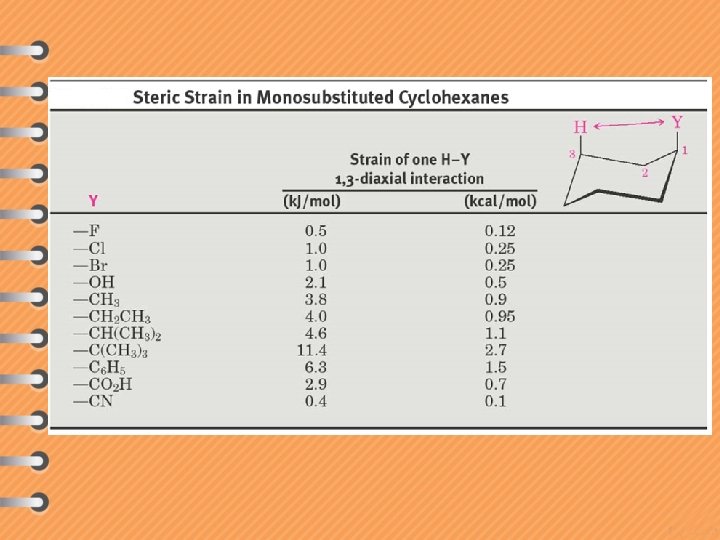
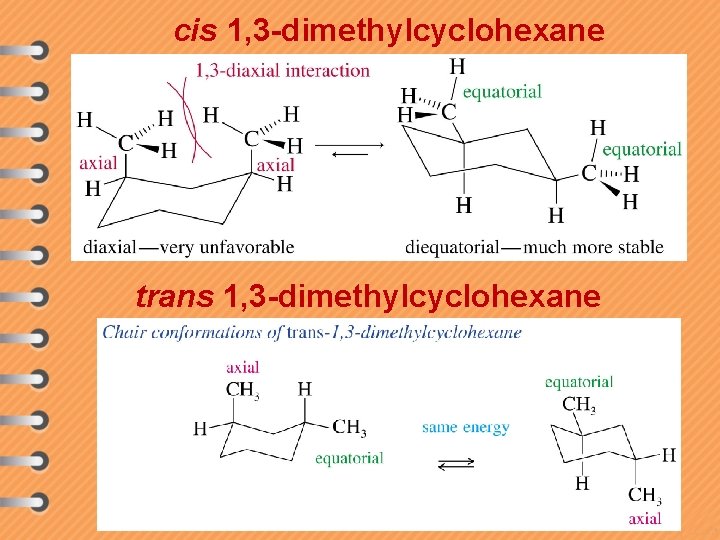
cis 1, 3 -dimethylcyclohexane trans 1, 3 -dimethylcyclohexane
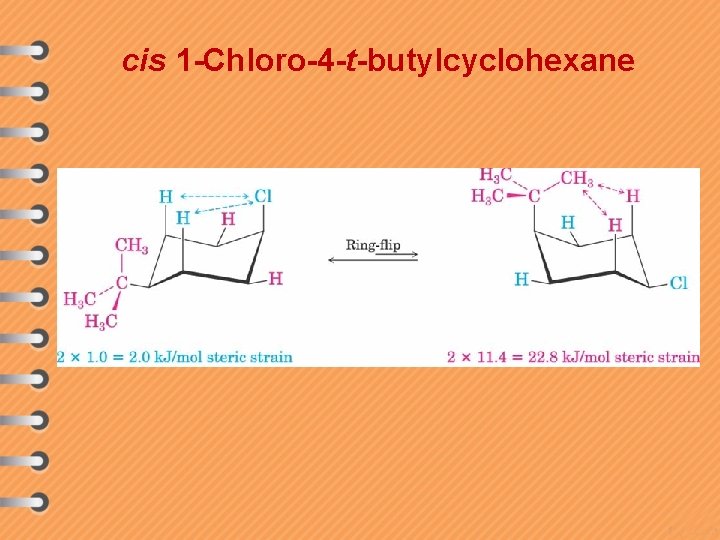
cis 1 -Chloro-4 -t-butylcyclohexane
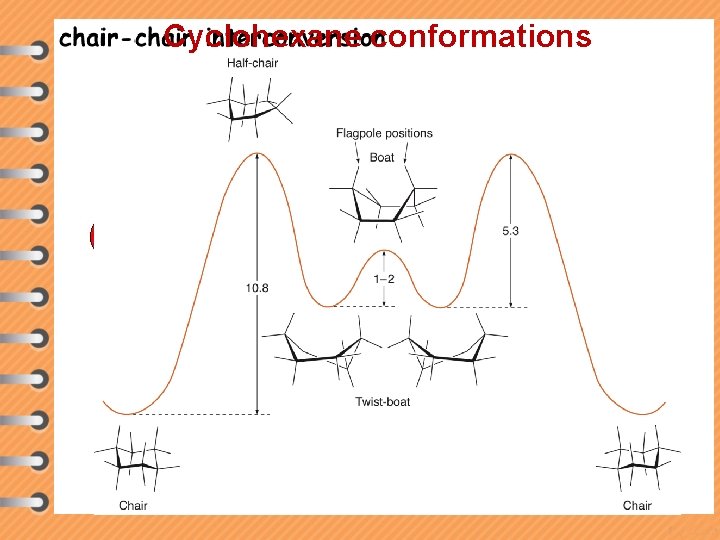
Cyclohexane conformations
Methods for determining conformations A number of methods have been used to determine configuration; v X-ray and electron diffraction, IR, Raman, UV, NMR spectra, photoelectron spectroscopy, v Optical Rotatory Dispersion (ORD) and Circular Dichroism (CD) measurements.
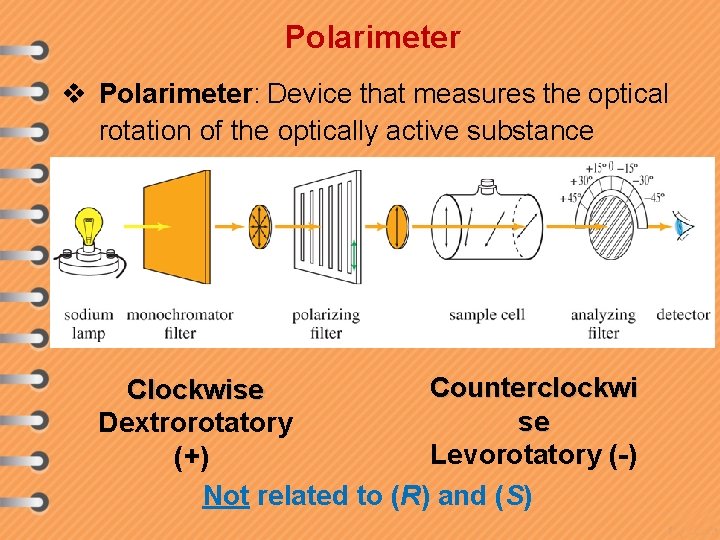
Polarimeter v Polarimeter: Device that measures the optical rotation of the optically active substance Counterclockwi Clockwise se Dextrorotatory Levorotatory (-) (+) Not related to (R) and (S)
![Specific Rotation [ ]D It is defined as the number of degrees of rotation Specific Rotation [ ]D It is defined as the number of degrees of rotation](http://slidetodoc.com/presentation_image_h2/8a23c4e774874656c184534cfbfd5f43/image-81.jpg)
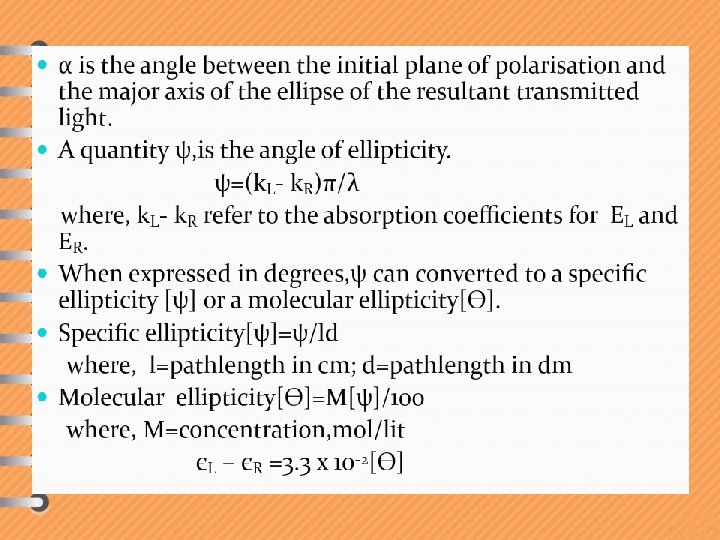
Specific Rotation [ ]D It is defined as the number of degrees of rotation caused by a solution of 1. 0 g of compound per ml of solution taken in a polarimeter tube 1. 0 dm (10 cm) long at a specific temperature and wavelength. The specific rotation is calculated from observed angle of rotation, as below: Specific rotation calculated in this way is a physical property of an optically active substance. You always get the same a = observed rotation value of c = concentration ( g/m. L ) l = length of cell ( dm ) D = yellow light from sodium lamp (5893 Å) t = temperature ( Celsius )
Chiral structure can be distinguished and characterized by polarized light v Optical rotation: the rotation polarized light by the sample of linearly v Optical Rotatory Dispersion (ORD): the variation of optical rotation as a function of wavelength. The spectrum of optical rotation. v Circular Dichroism (CD): the difference in absorption of left and right circularly light.

v Dichroism is used to denote direction-dependent light absorption. v Circular Dichroism (CD) The production of an elliptically polarized wave when a linearly polarized light wave passes through a substance that has differences in the extinction coefficients for left- and right-handed polarized light. v Birefringence refers to the direction-dependent index of refraction v Circular birefringence A phenomenon in which there is a difference between the refractive indices of the molecules of a substance for right and leftcircularly polarized light
Optical rotation v When a plane polarized light (PPL) is passed through optically active compound due to it’s circular birefringence results in unequal rate of propagation of left and right circularly polarized rays. v This unequal rate of propagation of both left and right circularly polarized light deviates the PPL from it’s original direction and it is called as optical rotation v Optical rotation caused by compound changed with wavelength of light was first noted by Biot in 1817.
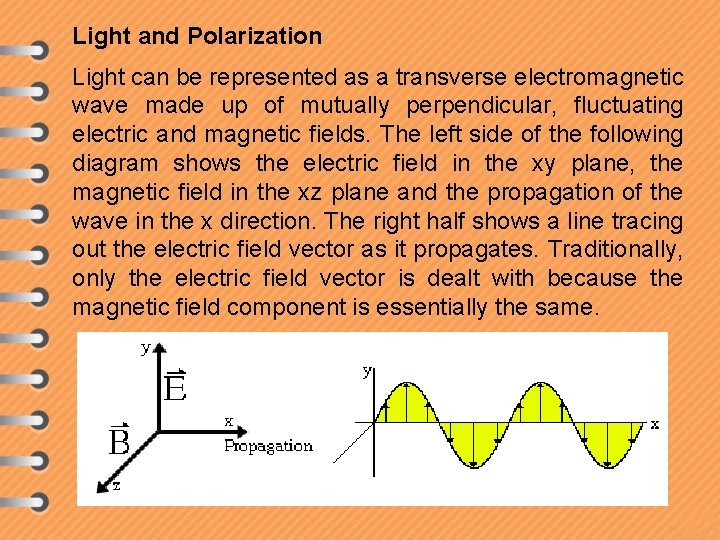
Light and Polarization Light can be represented as a transverse electromagnetic wave made up of mutually perpendicular, fluctuating electric and magnetic fields. The left side of the following diagram shows the electric field in the xy plane, the magnetic field in the xz plane and the propagation of the wave in the x direction. The right half shows a line tracing out the electric field vector as it propagates. Traditionally, only the electric field vector is dealt with because the magnetic field component is essentially the same.
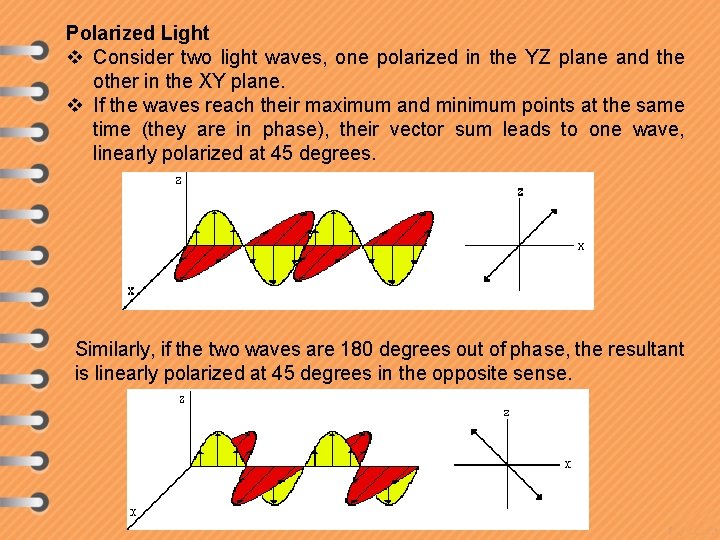
Polarized Light v Consider two light waves, one polarized in the YZ plane and the other in the XY plane. v If the waves reach their maximum and minimum points at the same time (they are in phase), their vector sum leads to one wave, linearly polarized at 45 degrees. Similarly, if the two waves are 180 degrees out of phase, the resultant is linearly polarized at 45 degrees in the opposite sense.
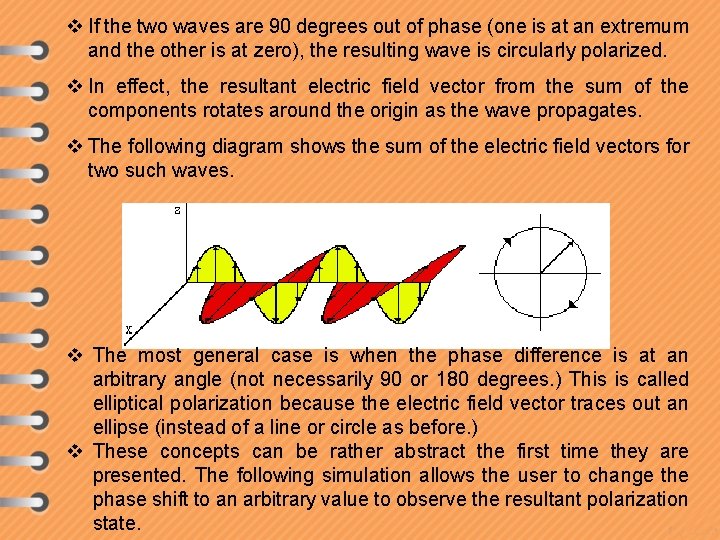
v If the two waves are 90 degrees out of phase (one is at an extremum and the other is at zero), the resulting wave is circularly polarized. v In effect, the resultant electric field vector from the sum of the components rotates around the origin as the wave propagates. v The following diagram shows the sum of the electric field vectors for two such waves. v The most general case is when the phase difference is at an arbitrary angle (not necessarily 90 or 180 degrees. ) This is called elliptical polarization because the electric field vector traces out an ellipse (instead of a line or circle as before. ) v These concepts can be rather abstract the first time they are presented. The following simulation allows the user to change the phase shift to an arbitrary value to observe the resultant polarization state.
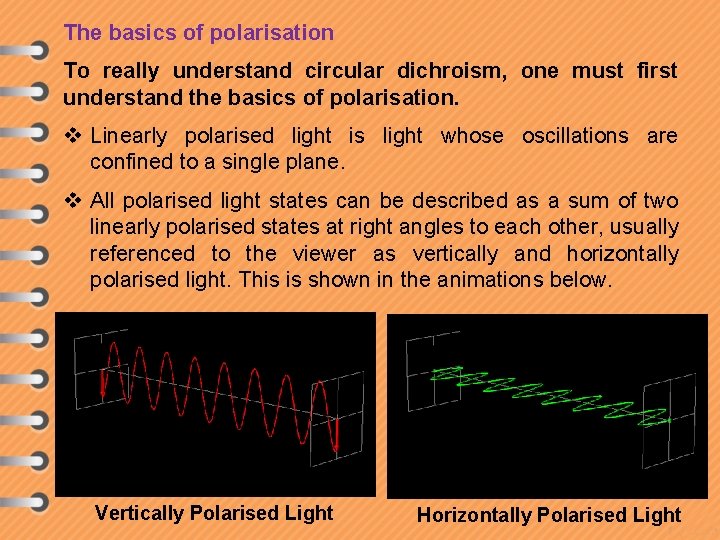
The basics of polarisation To really understand circular dichroism, one must first understand the basics of polarisation. v Linearly polarised light is light whose oscillations are confined to a single plane. v All polarised light states can be described as a sum of two linearly polarised states at right angles to each other, usually referenced to the viewer as vertically and horizontally polarised light. This is shown in the animations below. Vertically Polarised Light Horizontally Polarised Light
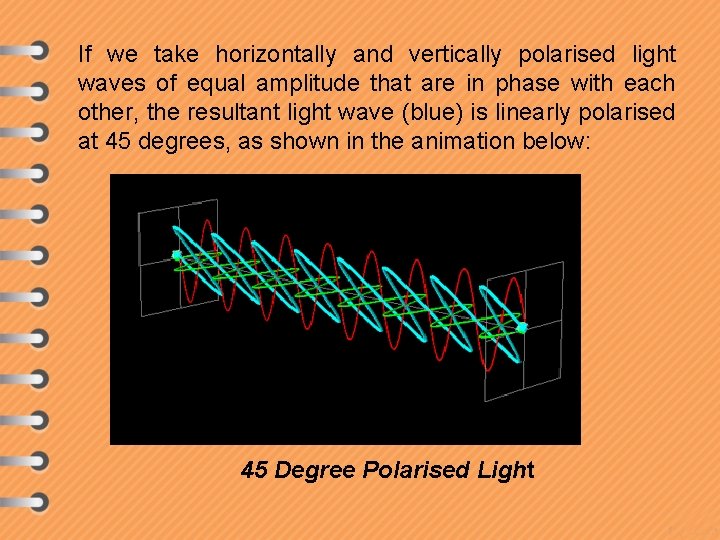
If we take horizontally and vertically polarised light waves of equal amplitude that are in phase with each other, the resultant light wave (blue) is linearly polarised at 45 degrees, as shown in the animation below: 45 Degree Polarised Light
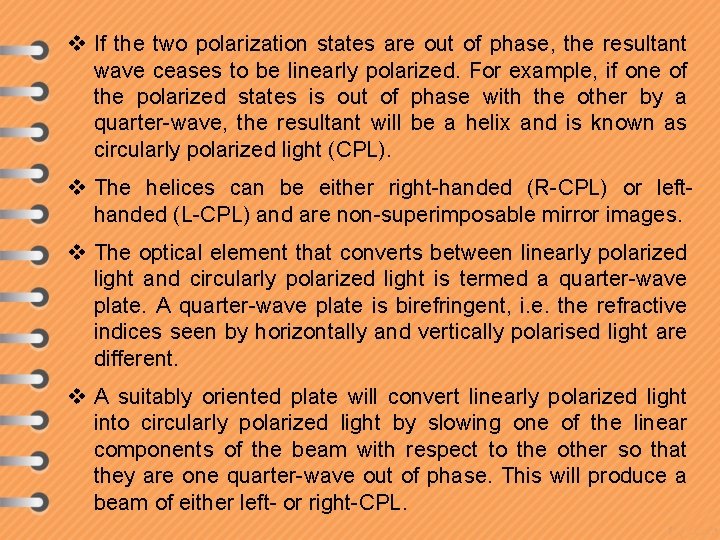
v If the two polarization states are out of phase, the resultant wave ceases to be linearly polarized. For example, if one of the polarized states is out of phase with the other by a quarter-wave, the resultant will be a helix and is known as circularly polarized light (CPL). v The helices can be either right-handed (R-CPL) or lefthanded (L-CPL) and are non-superimposable mirror images. v The optical element that converts between linearly polarized light and circularly polarized light is termed a quarter-wave plate. A quarter-wave plate is birefringent, i. e. the refractive indices seen by horizontally and vertically polarised light are different. v A suitably oriented plate will convert linearly polarized light into circularly polarized light by slowing one of the linear components of the beam with respect to the other so that they are one quarter-wave out of phase. This will produce a beam of either left- or right-CPL.
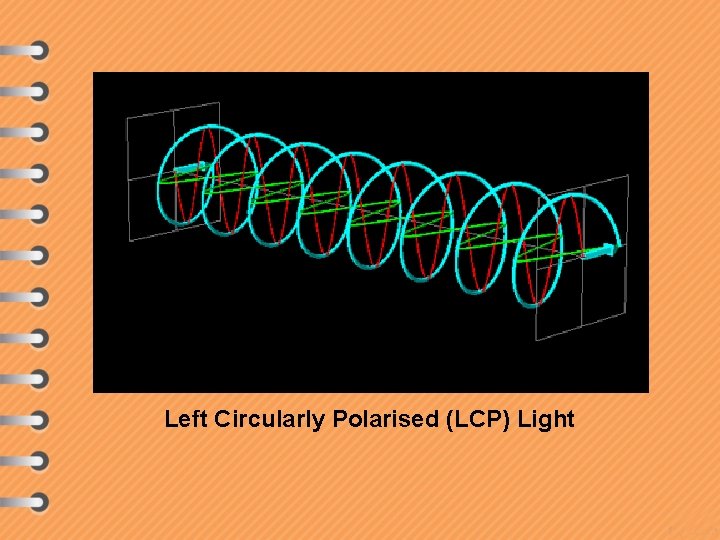
Left Circularly Polarised (LCP) Light
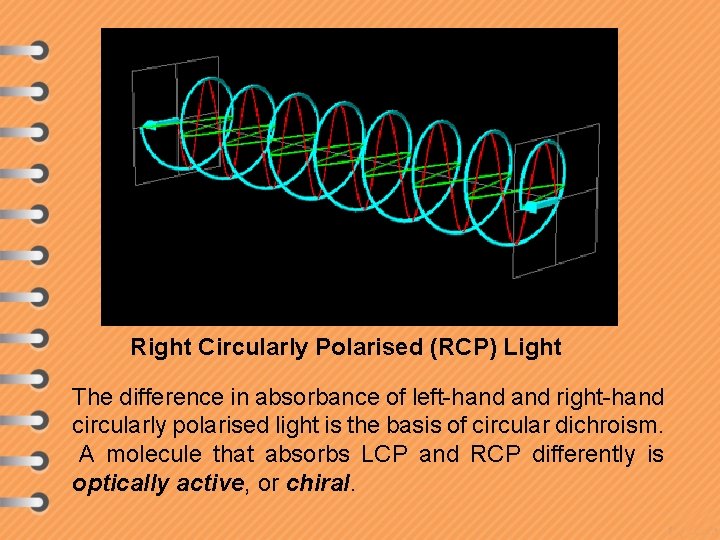
Right Circularly Polarised (RCP) Light The difference in absorbance of left-hand right-hand circularly polarised light is the basis of circular dichroism. A molecule that absorbs LCP and RCP differently is optically active, or chiral.
When a ray of monochromatic polarized light strikes a solution, several phenomenon’s occurs like: 1. Reflection on the surface 2. Refraction 3. Rotation of plane polarization 4. Absorption v Enantiomers are optically active compounds. v Optically active molecules have different refractive indices, and different extinction coefficients for L and R circularly polarized light.
Optical Rotatory dispersion (ORD) v ORD is defined as the rate of change of specific rotation or rotatory power with change in wavelength. v Light is an electromagnetic radiation and consist of vibrating electric and magnetic vector perpendicular to each other. v ORD curves in recent years are made use in structural determination by comparing the curve obtain from compound believed to have related structures particularly applied to carbonyl compounds.
v E. g. . ORD curves have been used to locate the position of carbonyl groups in steroid molecules. v Rotatory dispersion curves (i. e. plot of optical rotation against wavelength. ) can be classified into two main types. 1. Plain curves 2. Cotton effect curves.
Rotation of plane polarized light (Fresnel’s explanation) -: v According to Fresnel, a plane polarized light may be considered as the combination of two circularly polarized light of which one is right circularly polarized light (RCPL) & other is left circularly polarized light (LCPL) which are in equal & opposite in nature. v A circularly polarized light (CPL) is one whose plane of polarization rotates continuously & in the same sense around the axis of the polarization of the wave & it may be described as right handed screw or helix twisting around the direction of propagation, where LCPL wave describe the left handed screw.
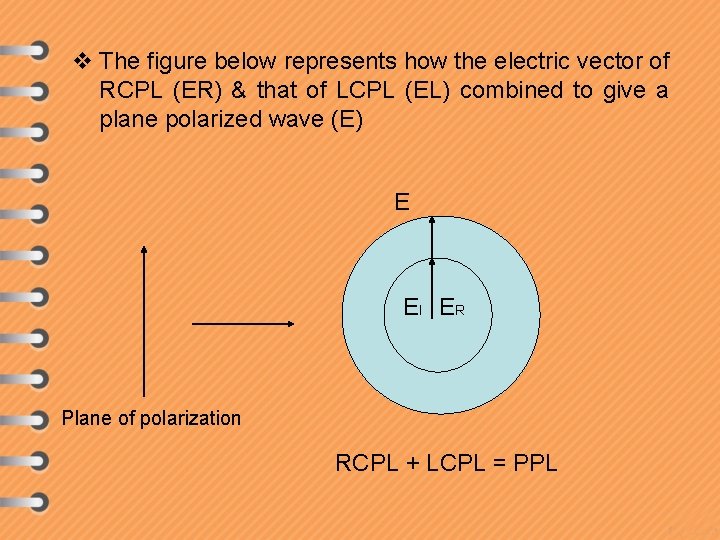
v The figure below represents how the electric vector of RCPL (ER) & that of LCPL (EL) combined to give a plane polarized wave (E) E El ER Plane of polarization RCPL + LCPL = PPL
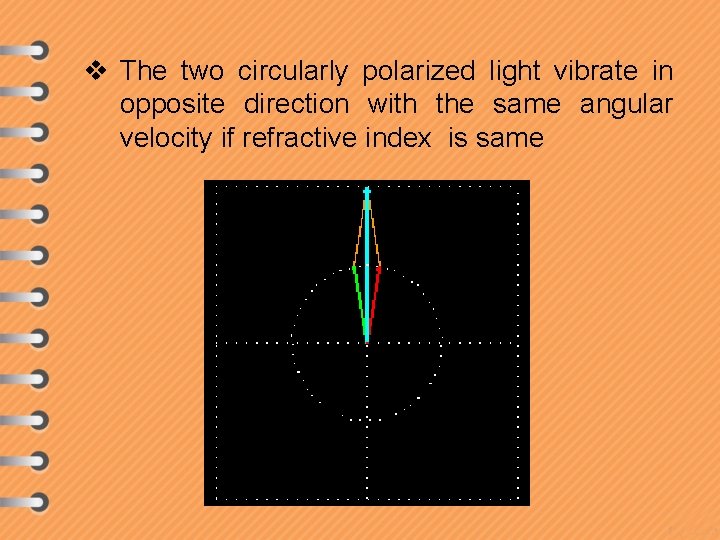
v The two circularly polarized light vibrate in opposite direction with the same angular velocity if refractive index is same
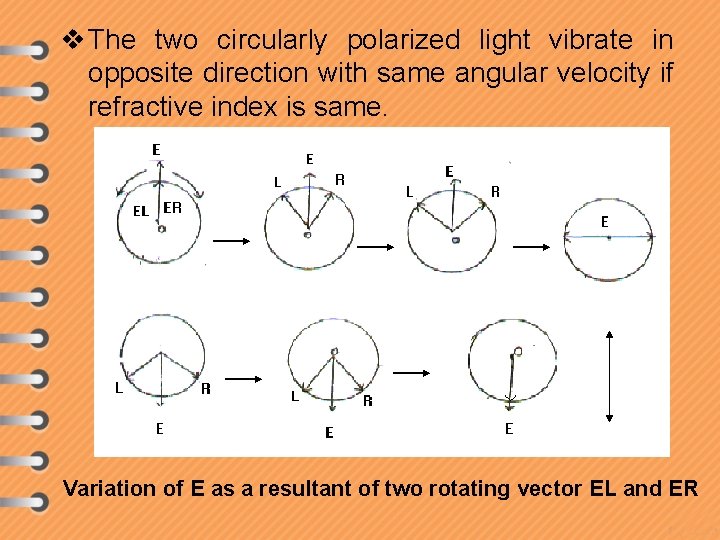
v The two circularly polarized light vibrate in opposite direction with same angular velocity if refractive index is same. Zero resultant Variation of E as a resultant of two rotating vector EL and ER
Specific rotation (Rotatory power): v It is the rotation produced by a solution in 10 cm length tube having 1 gm of substance in 100 ml. denoted by [ α ] v The specific rotation depends on following factors: Ø Ø Ø Nature of substance. Length of the column. Conc. of the sol. Temp of the sol. Nature of the solvent. Wavelength of the light used.
The angle of rotation per unit path length is, α = (n. L – n. R ) π / λ Where, λ = wavelength of incident light n = refractive index v If RCPL travels faster α medium is dextrorotatory, is positive & the v If LCPL travels faster then α is negative & the medium is levorotatory.

Cotton effect v The combination of circular dichroism and circular birefringence is known as cotton effect. Which may be studied by observing the change of optical rotation with the wavelength so called ORD. v It was discovered by a French physicist A. COTTON. v The curves obtained by plotting optical rotation v/s wavelength down to about 220 nm using photoelectric spectropolarimeters, known as ORD curves or Cotton effects.
v The absolute magnitude of the optical rotation at first varies rapidly with , crosses zero at absorption maxima and then again varies rapidly with but in opposite direction, this is known as Cotton effect and the curves describing such effect is called Cotton effect curves. They are of two types: 1. Plain curves 2. Anomalous curves (a) Single cotton effect curves (b) Multiple cotton effect curves
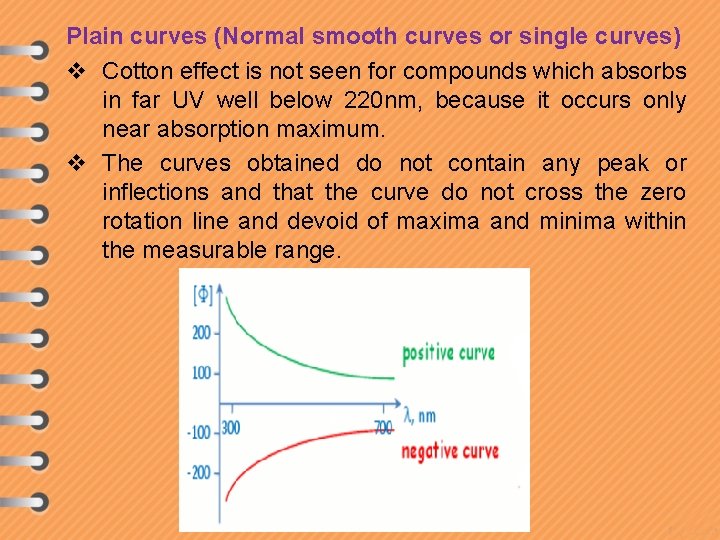
Plain curves (Normal smooth curves or single curves) v Cotton effect is not seen for compounds which absorbs in far UV well below 220 nm, because it occurs only near absorption maximum. v The curves obtained do not contain any peak or inflections and that the curve do not cross the zero rotation line and devoid of maxima and minima within the measurable range.
Anomalous curves v These curves on the other hand shows a number of extreme peaks and troughs depending on the number of absorbing groups and therefore known as Anomalous dispersion of optical rotation. v This type of curves is obtained for the compounds which contain an asymmetric carbon atom and also contain chromophore, which absorb near the UV region.

Single cotton effect curves v These are anomalous dispersion curves which shows maximum and minimum both of them occurring in the region of maximum absorption. v If in approaching the region of cotton effect from the long wavelength, one passes first through maximum (peak) and then a minimum (trough), the cotton effect is said to be positive.
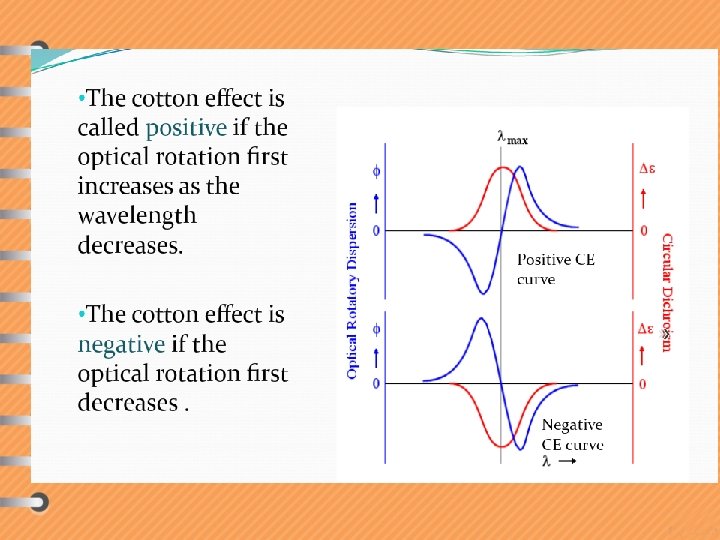
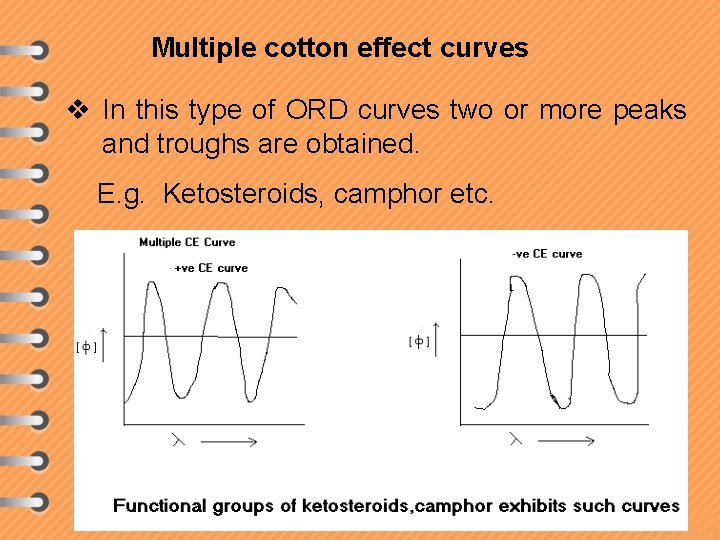
Multiple cotton effect curves v In this type of ORD curves two or more peaks and troughs are obtained. E. g. Ketosteroids, camphor etc.
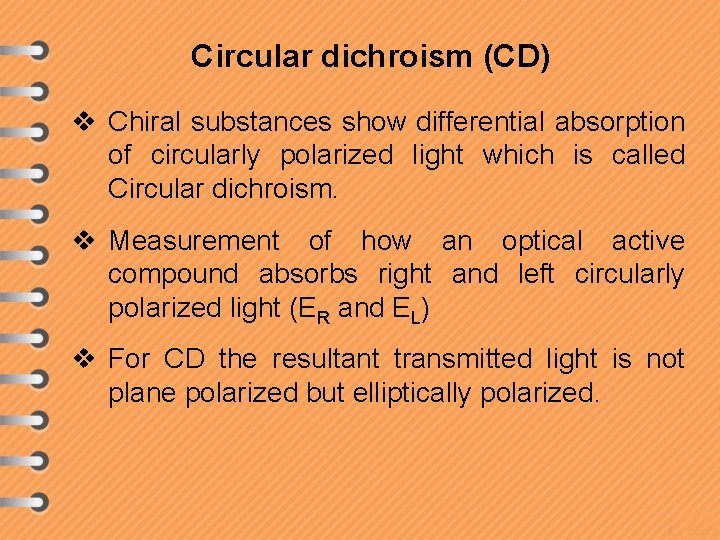
Circular dichroism (CD) v Chiral substances show differential absorption of circularly polarized light which is called Circular dichroism. v Measurement of how an optical active compound absorbs right and left circularly polarized light (ER and EL) v For CD the resultant transmitted light is not plane polarized but elliptically polarized.

Circular dichroism
Applications of CD v Determination of secondary structure of proteins that cannot be crystallized v Investigation of the effect of e. g. drug binding on protein secondary structure v Dynamic processes, e. g. protein folding v Studies of the effects of environment on protein structure v Secondary structure membrane proteins and super-secondary structure of v Study of ligand-induced conformational changes v Carbohydrate conformation v Investigations of protein-protein and protein-nucleic acid interactions v Folding recognition
Difference between ORD & CD v Plane polarized light v Circularly polarized light v Dispersive phenomena v Absorptive phenomena v Plane polarized is used and is not converted to elliptical light v Circular polarized is used and is converted to elliptical v Graphs are obtained by specific rotation vs wavelength v Graphs are obtained molar ellipicity vs wavelength
- Slides: 113