Steps of the scientific method 1 2 3


























- Slides: 36

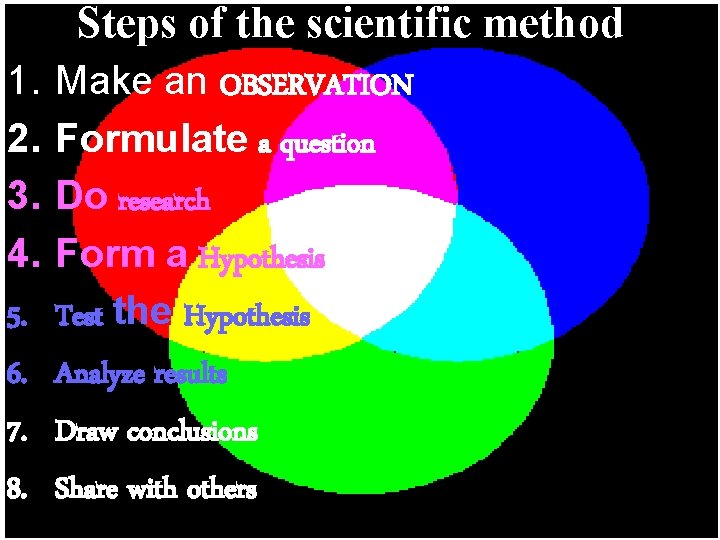
Steps of the scientific method 1. 2. 3. 4. 5. 6. 7. 8. Make an OBSERVATION Formulate a question Do research Form a Hypothesis Test the Hypothesis Analyze results Draw conclusions Share with others

DEMONSTRATION QUESTIONS WHAT ARE THE TWO LIQUIDS IN THE BEAKERS? WHAT WOULD BE A HYPOTHESIS FOR THIS EXPERIMENT? HOW CAN WE TEST OUR HYPOTHESIS? WHAT CONCLUSION CAN BE DRAWN FROM THIS DEMONSTATION?

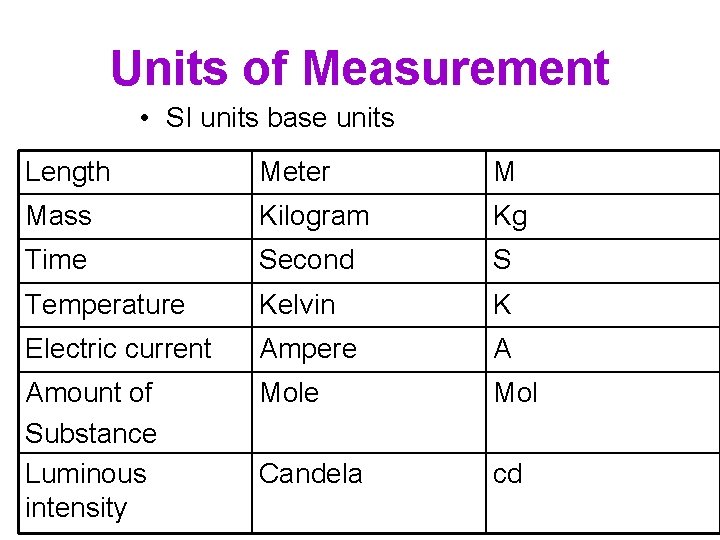
Units of Measurement • SI units base units Length Meter M Mass Kilogram Kg Time Second S Temperature Kelvin K Electric current Ampere A Amount of Substance Luminous intensity Mole Mol Candela cd

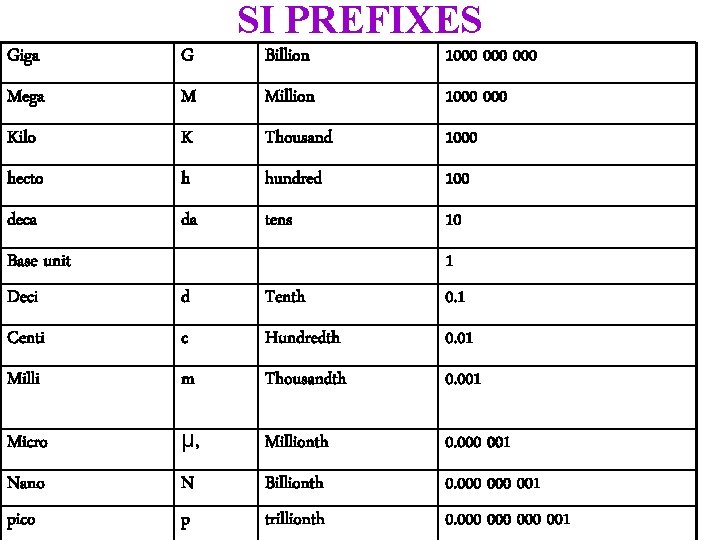
SI PREFIXES Giga G Billion 1000 000 Mega M Million 1000 Kilo K Thousand 1000 hecto h hundred 100 deca da tens 10 Base unit Deci d Tenth 1 0. 1 Centi c Hundredth 0. 01 Milli m Thousandth 0. 001 Micro µ, Millionth 0. 000 001 Nano pico N p Billionth trillionth 0. 000 001 0. 000 000 001

PRACTICE CONVERSIONS 1. 113 g to milligrams 2. 700 micrometers to nanometers 3. 49, 890 m to centimeters

What is chemistry The study of matter and how it changes Example of chemistry: soap food, glass Because of what it is made of or

What is matter? Anything that has mass and occupies space

BASIC MATTER can be broken down into… 1. ATOM: SMALLEST PARTICLE THAT HAS PROPERTIES OF AN ELEMENT 2. ELEMENT: SUBSTANCE THAT CANNOT BE BROKEN DOWN INTO A SIMPLER SUBSTANCE 3. COMPOUND: SUBSTANCE MADE OF ATOMS OF MORE THAN 1 ELEMENT BOUND TOGETHER

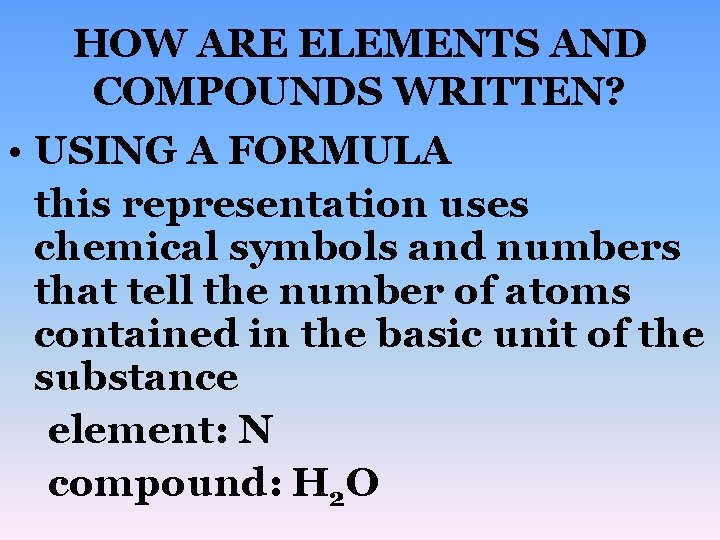
HOW ARE ELEMENTS AND COMPOUNDS WRITTEN? • USING A FORMULA this representation uses chemical symbols and numbers that tell the number of atoms contained in the basic unit of the substance element: N compound: H 2 O

What is the smallest unit of a substance that exhibits all of the properties characteristic of that substance? Molecule

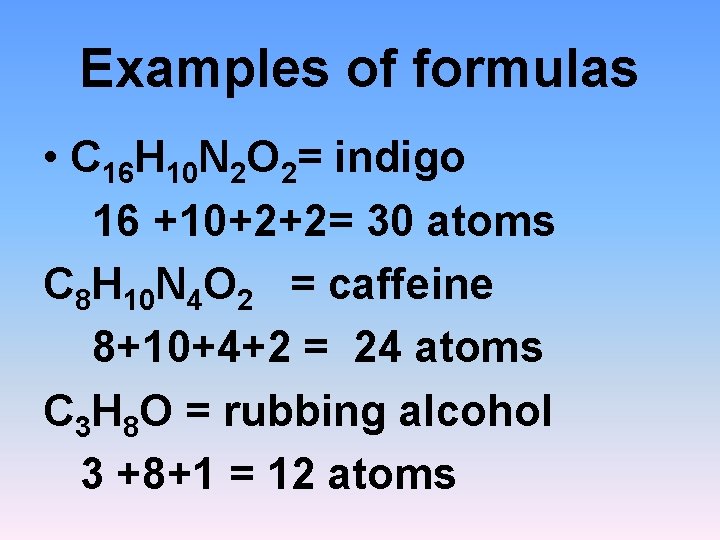
Examples of formulas • C 16 H 10 N 2 O 2= indigo 16 +10+2+2= 30 atoms C 8 H 10 N 4 O 2 = caffeine 8+10+4+2 = 24 atoms C 3 H 8 O = rubbing alcohol 3 +8+1 = 12 atoms

What is the difference between a pure substance and a mixture? • Pure: matter with a fixed composition and definite properties, can’t be separated or broken down by physical actions such as boiling grinding or melting represented by a chemical formula C 6 H 12 O 6 Mixture: a combination of more than one pure substance air, grape juice

Matter and Energy What is the kinetic theory? 1. All matter is made of atoms and molecules that act like tiny particles 2. Tiny particles are always in motion the higher the temperature, the faster the particles move 3. At the same temperatures, the more massive particles move slower than the less massive particles

What are the common states of matter? 1. Solid: fixed position, rigid, can vibrate, closely packed 2. liquid: closely packed but can slide past each other 3. gas: constant state of motion, rarely stick together 4. plasma: fast moving charged particles

Solid: fixed position, rigid, can vibrate, closely packed 2. liquid: closely packed but can slide past each other gas: constant state of motion, rarely stick together 4. plasma: fast moving charged particles

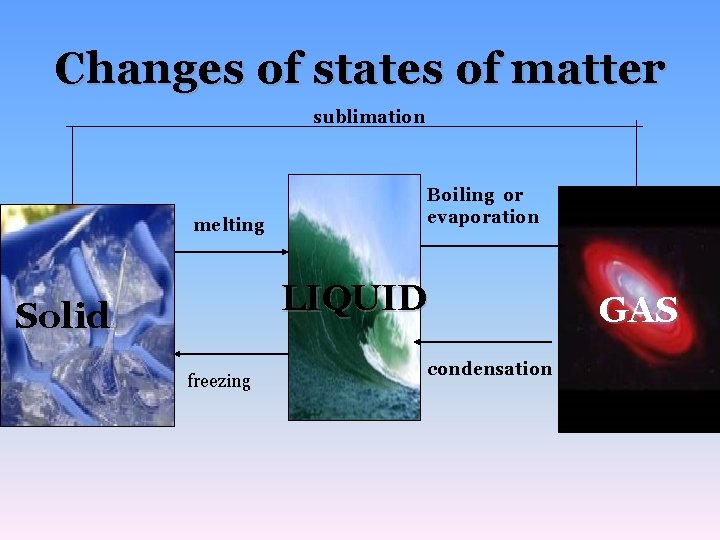
What is energy? • Ability to change or move matter What does changing states of matter do to a substance? • changes attractive forces between molecules, doesn’t change composition of a substance

State the law of conservation of mass? • Mass can not be created or destroyed State the law of conservation of energy? • Energy can not be created or destroyed.

Changes of states of matter sublimation melting Boiling or evaporation LIQUID Solid freezing condensation GAS

What is a chemical property of a substance? The way a substance reacts with others to form new substances with different properties by either combining with other elements or by breaking apart into new substances (corrosive, reactive, flammable) ex mercury…toxic steel…… reactive with oxyen to form rust sodium …. Flammable in O 2

What is reactivity? Ability of a substance to combine chemically with another What is flammability? Chemical property that describes whether substances will react in the presence of O 2 and burn when exposed to flame

What are physical properties? Characteristics of a substance that can be observed or measured without changing the composition of the substance Remain constant for specific pure substances Shape, color, odor, texture, melting point, boiling point, strength, hardness, ability to conduct electricity or heat, magnetism, density Ex mercury is liquid at room temperature

How to classify mixtures? By how thoroughly the substances mix together. Mix solid + liquid…. Homogeneous mixture clear and mixing occurs between the individual units and is the same throughout salt + water= salt water Heterogeneous cloudy mixture and mixture between substances isn’t uniformly mixed flour + water

Liquid + liquid Miscible Describes two or more liquids that are able to dissolve into each other in various proportions gasoline Immiscible Describes two or more liquid that do not mix into each other. oil and water

DENSITY: physical property D= m/V density equals mass divided volume Buoyancy: the force with which a more dense fluid pushes a less dense substance upward

What is a chemical change? A change that happens when a substance changes composition by forming one or more new substances cellular respiration photosynthesis combustion clues: energy released, smell or smoke

A change What is a physical change? in the physical form or properties of a substance that occurs without a change in composition Ex: melting, freezing, evaporating, grinding, dissolving,

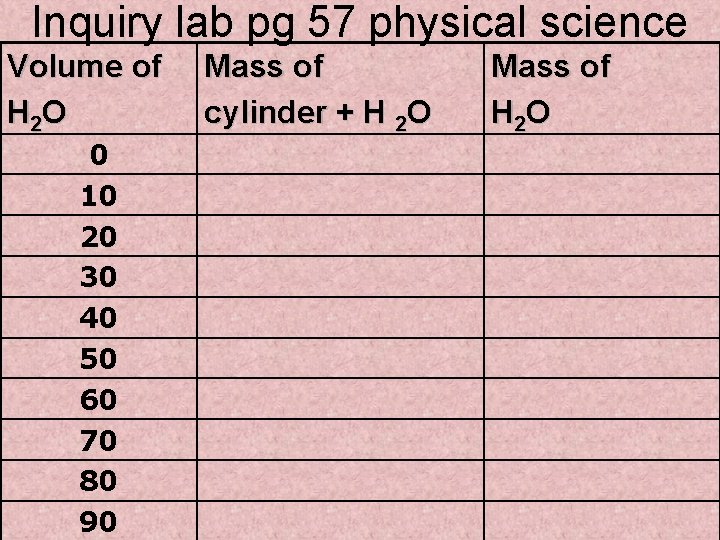
Inquiry lab pg 57 physical science Volume of H 2 O 0 10 20 30 40 50 60 70 80 90 Mass of cylinder + H 2 O Mass of H 2 O

What is electron affinity? v. The attraction of an atom for an additional electron v. Metals have low affinity because they give away electrons v. Nonmetals have high affinity because they take on electrons v. The electron affinity tends to increase from left to right in the periodic table and from bottom to top

What are families/groups? q Elements grouped together by common properties. They fall in the same vertical column of the periodic table Column #1 alkali metals (+1 oxidation #) Column #2 alkaline earth metals(+2 oxid #) Column #3 -12 transition metals (many have multiple oxidation number-least reactive of the metals) Column #16 chalcogens (-2 oxidation #) Column #17 halogens (-1 oxidation #) most reactive nonmetals Column #18 noble gases (0 oxidation #) nonreactive due to filled outer energy level lanthanide series actinide series

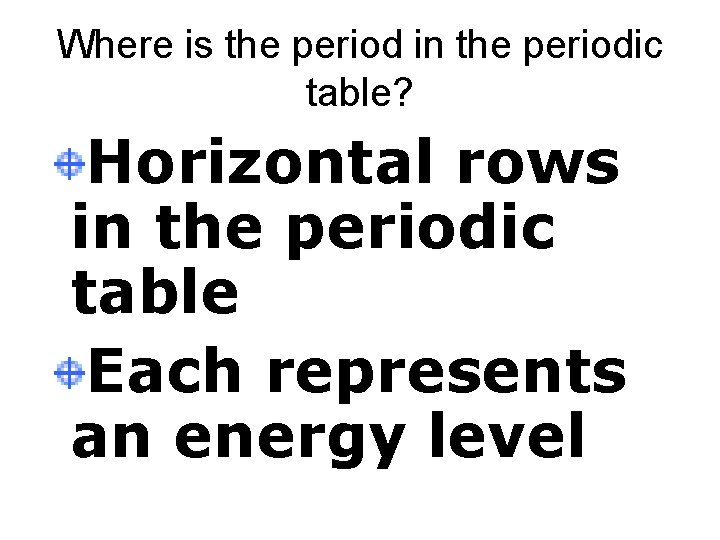
Where is the period in the periodic table? Horizontal rows in the periodic table Each represents an energy level

What are metals? • Groups of elements that has: Luster (shiny) Good conductors of heat and electricity 1 -3 valence electrons Located in the periodic table left of the metalloids excluding H Solids are room temperature(ex mercury)

What are metalloids? Elements that have properties of both metals and nonmetals. Located diagonally between the metals, nonmetals B, Si, Ge, As, Sb, Te,

What are nonmetals? Elements that do not have properties of metals Located on the right side of the periodic table Have more than 4 valence electrons Dull/lack luster Do not conduct heat or electricity well Typically gases or brittle solids at room temperature

What is a subscript? • Small lowered number after the symbol for an element that indicates the number of atoms of the element present in the formula of the molecule • Subscripts of one in a symbol are understood and therefore not shown • Example O 2 or Na. Cl or K 2 SO 4

What is ionization energy Energy needed to remove an electron from an atom. Ionization energy tends to increase from left to right and decrease from top to bottom

Periodic trends of Elements Reactivity of metals increases down the periodic table and from right to left across the periodic table Reactivity of non metals increases up the periodic table and from left to right across the periodic table Atomic radius increases down the periodic table and form right to left across the periodic table