Steps for Balancing Equations 1 Write the skeleton


Steps for Balancing Equations 1. Write the skeleton equation Use arrows, +/- & physical states of matter 2. Count the atoms of the reactants 3. Count the atoms of the products 4. Change the coefficients to make the number of atoms of each element equal on both sides of the equation NEVER, NEVER 5. Write CHANGE THE SUBSCRIPT the coefficients in their lowest possible ratio
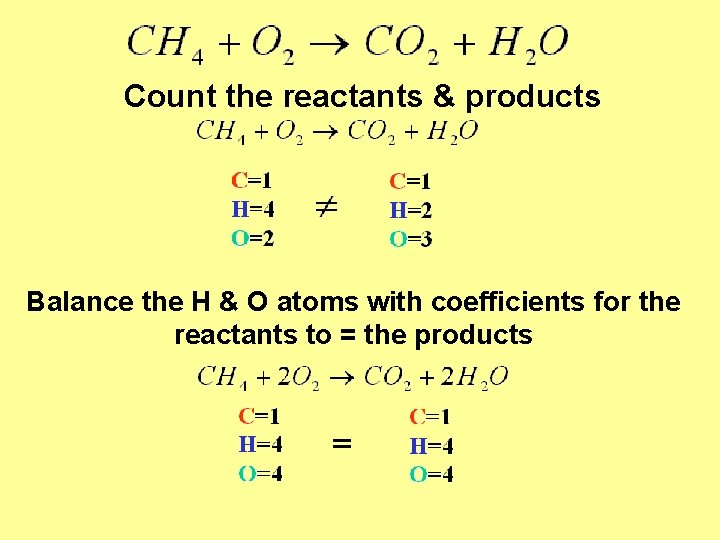
Count the reactants & products Balance the H & O atoms with coefficients for the reactants to = the products
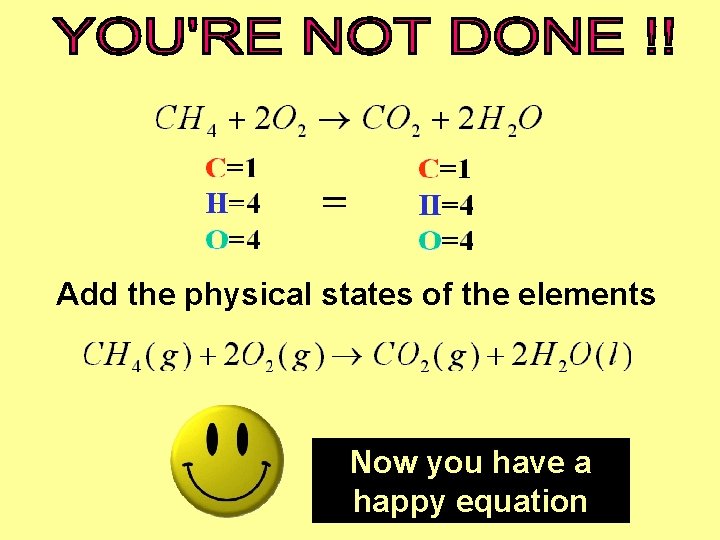
Add the physical states of the elements Now you have a happy equation
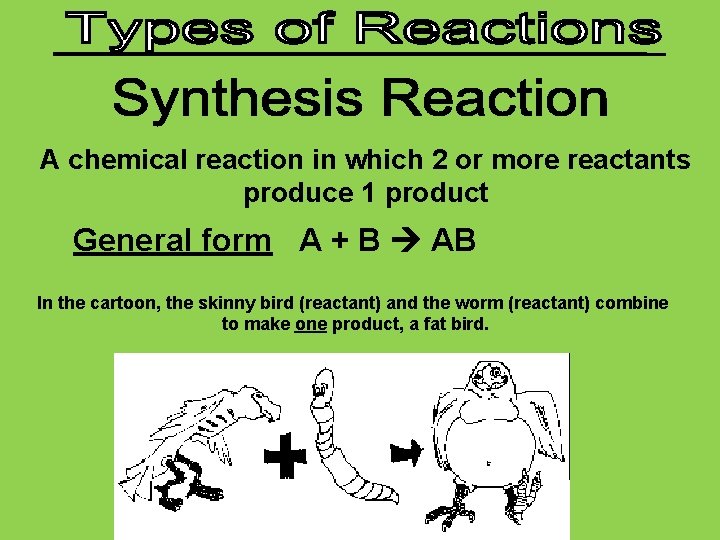
A chemical reaction in which 2 or more reactants produce 1 product General form A + B AB In the cartoon, the skinny bird (reactant) and the worm (reactant) combine to make one product, a fat bird.
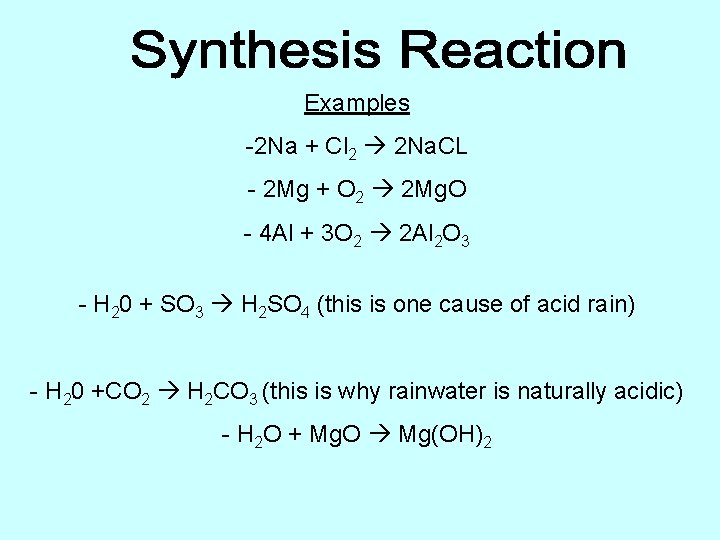
Examples -2 Na + Cl 2 2 Na. CL - 2 Mg + O 2 2 Mg. O - 4 Al + 3 O 2 2 Al 2 O 3 - H 20 + SO 3 H 2 SO 4 (this is one cause of acid rain) - H 20 +CO 2 H 2 CO 3 (this is why rainwater is naturally acidic) - H 2 O + Mg. O Mg(OH)2
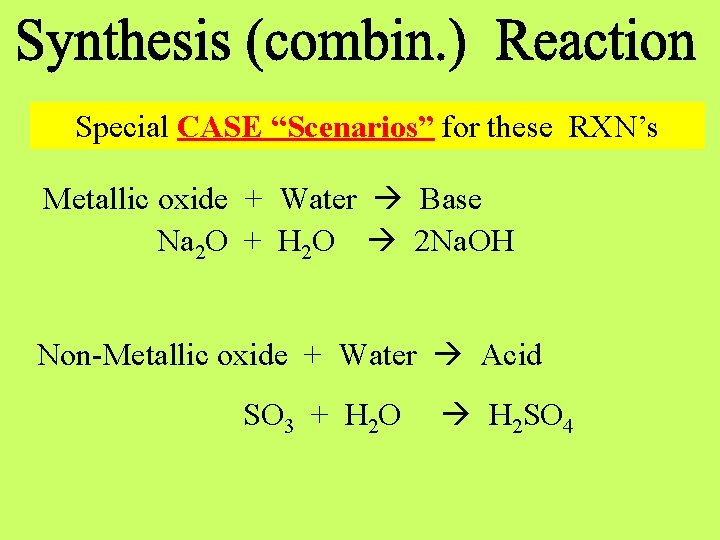
Special CASE “Scenarios” for these RXN’s Metallic oxide + Water Base Na 2 O + H 2 O 2 Na. OH Non-Metallic oxide + Water Acid SO 3 + H 2 O H 2 SO 4
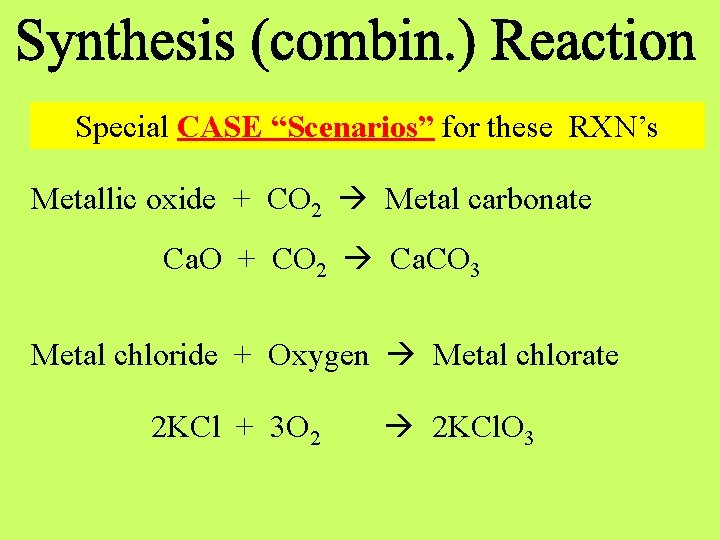
Special CASE “Scenarios” for these RXN’s Metallic oxide + CO 2 Metal carbonate Ca. O + CO 2 Ca. CO 3 Metal chloride + Oxygen Metal chlorate 2 KCl + 3 O 2 2 KCl. O 3
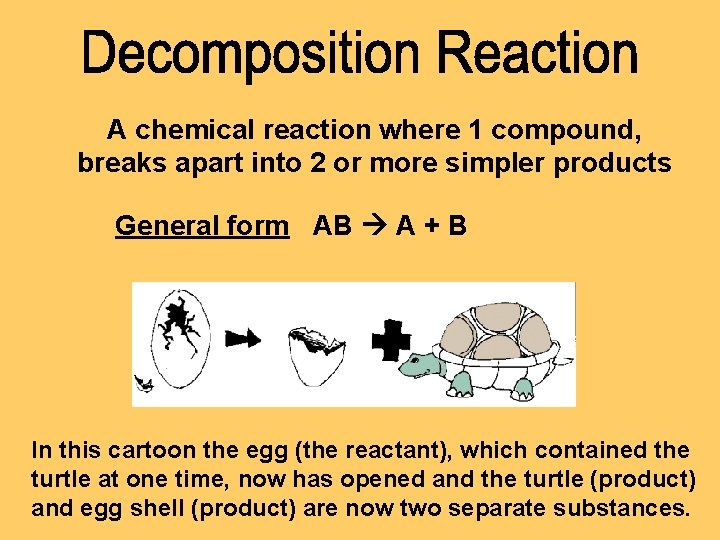
A chemical reaction where 1 compound, breaks apart into 2 or more simpler products General form AB A + B In this cartoon the egg (the reactant), which contained the turtle at one time, now has opened and the turtle (product) and egg shell (product) are now two separate substances.
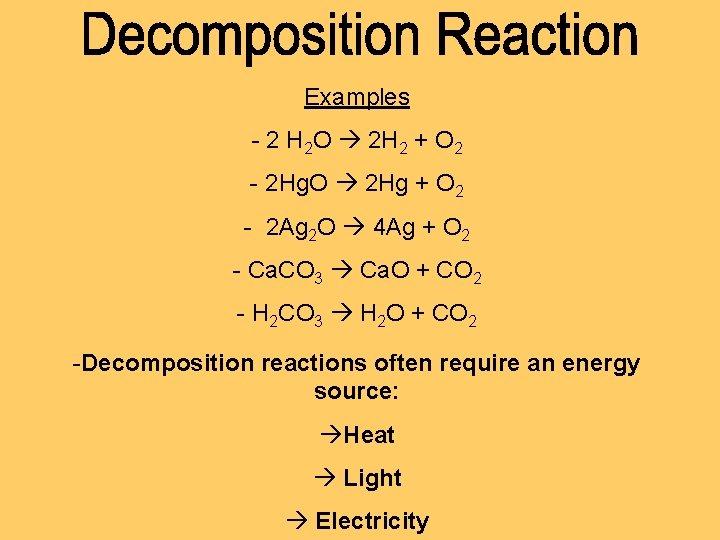
Examples - 2 H 2 O 2 H 2 + O 2 - 2 Hg. O 2 Hg + O 2 - 2 Ag 2 O 4 Ag + O 2 - Ca. CO 3 Ca. O + CO 2 - H 2 CO 3 H 2 O + CO 2 -Decomposition reactions often require an energy source: Heat Light Electricity
Special CASE “Scenarios” for these RXN’s Heating an acid non metallic oxide + water Heating a base metallic oxide + water Heating a carbonate oxide + CO 2 Heating a metal chlorate chloride + O 2 Heating a metallic oxide Metal + O 2 Heating a sulfite Metal oxide + SO 2 These are just the reverse of their synthesis RXN’s
A reaction in which atoms of one element replace the atoms of another element that is part of a compound Notice, the guy in the orange shirt steals the date of the other guy. So, a part of one of the reactants trades places and is in a different place among the products
1. Cations replace Cations General Form AB + C CB + A C would be the cation/metal and so replaced A, which would have to be a cation 2. Anions replace Anions General Form DE + F DF + E F would be the anion/non-metal and so replaced E, which would have to be a anion
Examples: a. Cu + Ag. NO 3 Cu(NO 3)2 + Ag Cation replaces Cation b. Na. I + Cl 2 Na. Cl + I 2 Anion replaces Anion

Special situations for SR RXN’s 1. Activity Series Definition A list of metal/cations in order of decreasing reactivity Used to determine if one metal can replace another in a RXN The higher its position on chart the more metals it can replace Ex: sodium will replace aluminum The lower its position on the chart the less metals it can replace Ex: zinc will not replace magnesium

Special situations for SR RXN’s 2. Water for writing RXN’s it may be useful to write water as H(OH). Why? 3. Halogens The halogens also have a series; As you go down group 17 they decrease in reactivity Ex: Chlorine cannot replace fluorine but it can replace bromine
Special situations for SR RXN’s Do the following reactions occur? Explain. Zn + H 2 SO 4 H 2 + Zn. SO 4 Sn + 2 Na. NO 3 Sn(NO 3)2 + 2 Na. Cl + F 2 2 Na. F + Cl 2 Ca. Cl 2 + I 2 Ca. I 2 + Cl 2
A reaction where there is an exchange of cations between 2 ionic compounds Notice how the first guy exchanged hats with the second guy, so they are both wearing each other's hat.
General Form AB + CD AD + CB ** Make sure of cation and anion placement Ex: Ba. Cl 2 + K 2 CO 3 Ba. CO 3 + 2 KCl 3 KOH + H 3 PO 4 3 H(OH) + K 3 PO 4
“Driving Forces” allow a RXN to take place Use your net ionic equation to see if one of the following are formed “Driving Forces” in double replacement RXN’s One of three things must form for these RXN’s to occur 1. A molecular compound like water forms 2. A gas forms that bubbles out i. e. (H 2, CO 2, H 2 S, CO, etc. ) 3. A precipitate forms
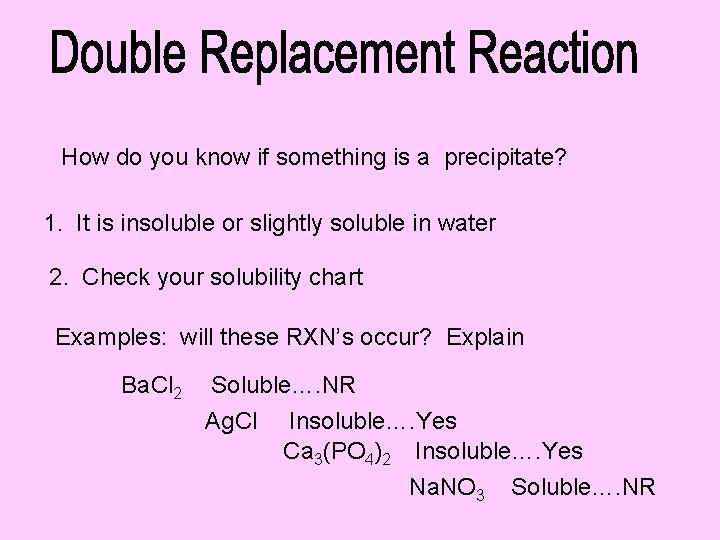
How do you know if something is a precipitate? 1. It is insoluble or slightly soluble in water 2. Check your solubility chart Examples: will these RXN’s occur? Explain Ba. Cl 2 Soluble…. NR Ag. Cl Insoluble…. Yes Ca 3(PO 4)2 Insoluble…. Yes Na. NO 3 Soluble…. NR
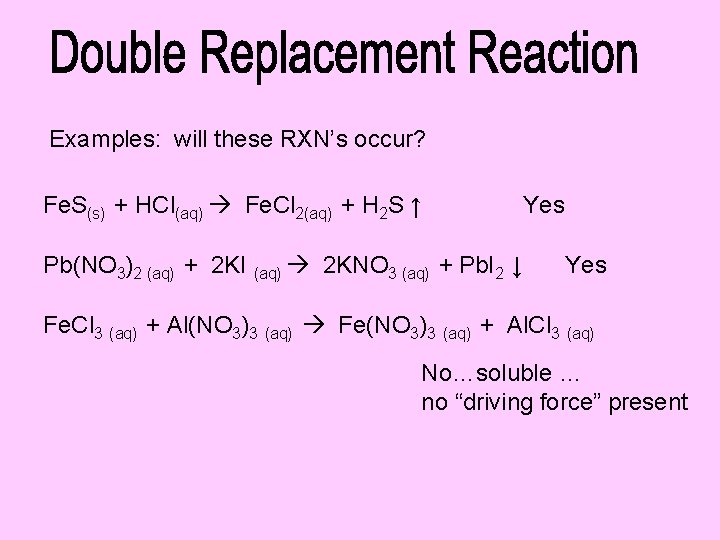
Examples: will these RXN’s occur? Fe. S(s) + HCl(aq) Fe. Cl 2(aq) + H 2 S ↑ Pb(NO 3)2 (aq) + 2 KI (aq) 2 KNO 3 (aq) + Pb. I 2 ↓ Yes Fe. Cl 3 (aq) + Al(NO 3)3 (aq) Fe(NO 3)3 (aq) + Al. Cl 3 (aq) No…soluble … no “driving force” present
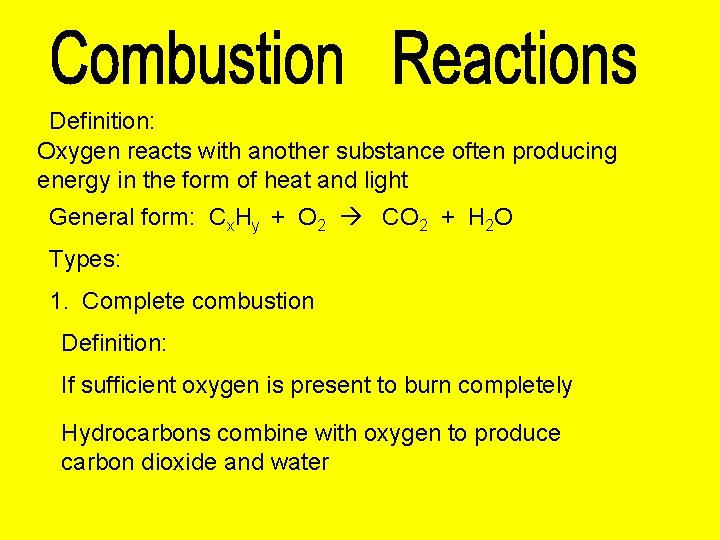
Definition: Oxygen reacts with another substance often producing energy in the form of heat and light General form: Cx. Hy + O 2 CO 2 + H 2 O Types: 1. Complete combustion Definition: If sufficient oxygen is present to burn completely Hydrocarbons combine with oxygen to produce carbon dioxide and water

2. Incomplete combustion Definition: Insufficient oxygen is present to burn completely Hydrocarbons combine with oxygen to produce poisonous carbon monoxide and solid elemental carbon as well as carbon dioxide and water Examples:

Extra “Special” RXN’s • Reactive metals and water (SR) • Group 1 and 2 metals react to form a metal hydroxide and hydrogen gas • Acid and a Base • React to form a salt (ionic compound composed of the anion of the acid and the cation of the base) and water
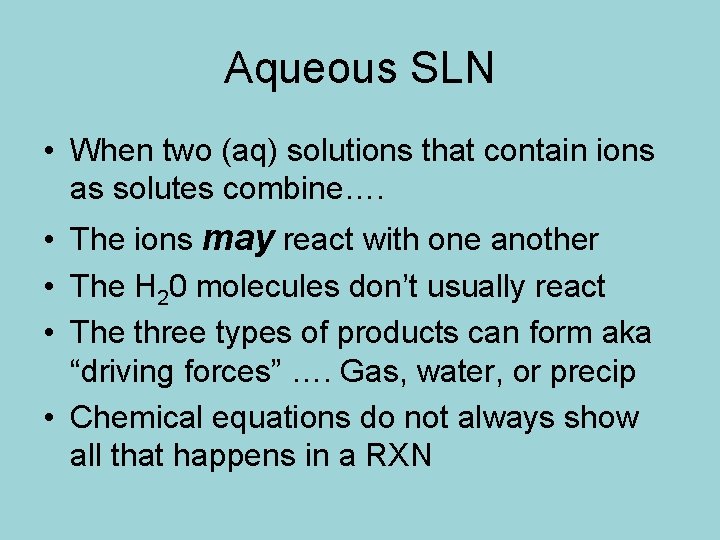
Aqueous SLN • When two (aq) solutions that contain ions as solutes combine…. • The ions may react with one another • The H 20 molecules don’t usually react • The three types of products can form aka “driving forces” …. Gas, water, or precip • Chemical equations do not always show all that happens in a RXN

Net Ionic Equations • • • Solutions are composed of Solute Solvent Aqueous SLN Is a sln in which the solvent is water
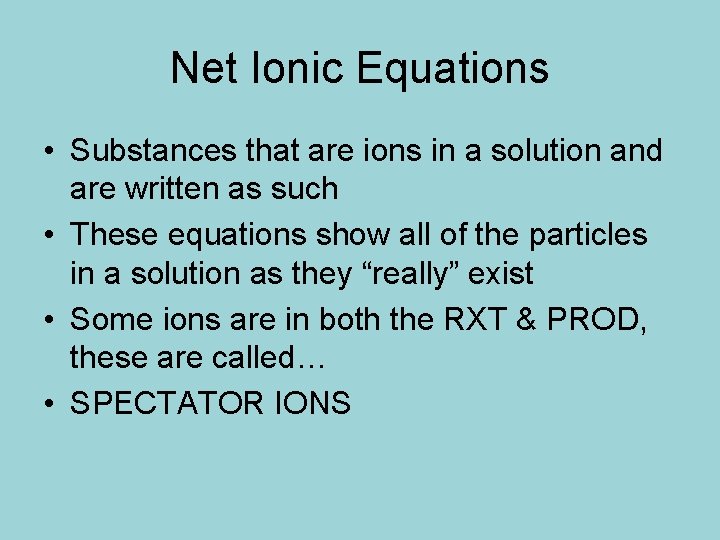
Net Ionic Equations • Substances that are ions in a solution and are written as such • These equations show all of the particles in a solution as they “really” exist • Some ions are in both the RXT & PROD, these are called… • SPECTATOR IONS

Net Ionic Equations • SPECTATOR IONS - dissociation only occurs if it is in an aqueous solution • S, L, G are not ions see your solubility chart
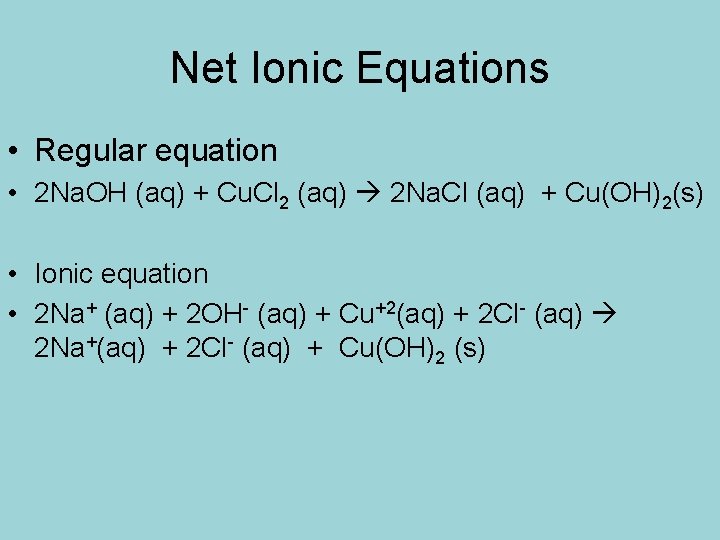
Net Ionic Equations • Regular equation • 2 Na. OH (aq) + Cu. Cl 2 (aq) 2 Na. Cl (aq) + Cu(OH)2(s) • Ionic equation • 2 Na+ (aq) + 2 OH- (aq) + Cu+2(aq) + 2 Cl- (aq) 2 Na+(aq) + 2 Cl- (aq) + Cu(OH)2 (s)
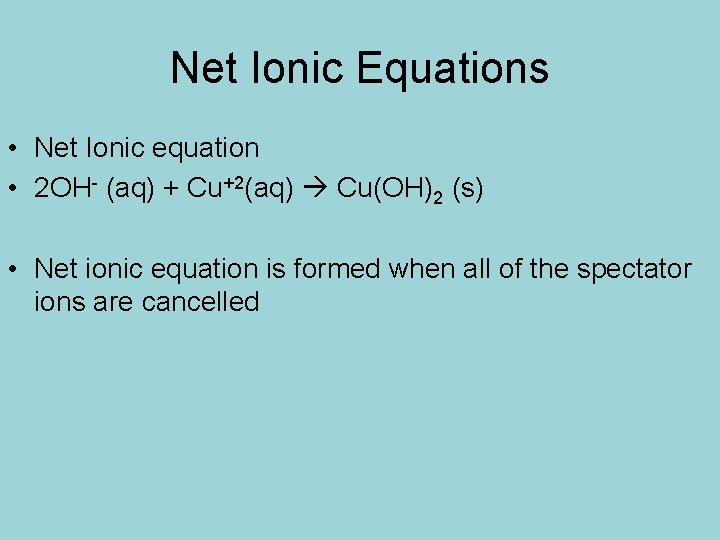
Net Ionic Equations • Net Ionic equation • 2 OH- (aq) + Cu+2(aq) Cu(OH)2 (s) • Net ionic equation is formed when all of the spectator ions are cancelled
- Slides: 31