Status of the CCR Continuity of Care Record













- Slides: 20

Status of the CCR: Continuity of Care Record Claudia Tessier, CAE, RHIA Co-Chair, ASTM E 31 Workgroup on CCR Executive Director, Mo. HCA Health Tech Net February 20, 2004

What Is the CCR? n Core data set of the most relevant and timely facts about a patient’s healthcare. n Organized and transportable. n Prepared by a practitioner at the conclusion of a healthcare encounter. n To enable the next practitioner to readily access such information. n May be prepared, displayed, and transmitted on paper or electronically.

Development of the CCR n Unique standards development effort n Consortium of sponsoring organizations n ASTM International n Massachusetts Medical Society n HIMSS n AAFP n AAP n Additional sponsoring organizations pending

CCR Will Benefit Healthcare Process Foster and improve continuity of care Enhance patient safety Reduce medical errors Reduce costs Enhance efficiency of health information exchange n Assure at least a minimum standard of health information transportability when patient is referred to, transferred to, or otherwise seen by another provider n n n

Why Is the CCR Needed? n CCR addresses the lack of appropriate, succinct, and up-to-date patient health information for practitioners at a new point of care. n CCR data is essential to good patient care and serves as a necessary bridge to a different environment, often with new practitioners who know little about the patient.

How Does the CCR Help Practitioners? n With the CCR the next healthcare practitioner can n Be informed about a patient’s allergies, medications, current and recent past diagnoses, most recent healthcare assessments and services, advance directives, and the recommendations of practitioners who last treated the patient. n More quickly and easily verify patient demographics and insurance status. n Minimize the effort to update patient’s most essential and relevant information in an EHR. n Reduce costs associated with the patient’s care.

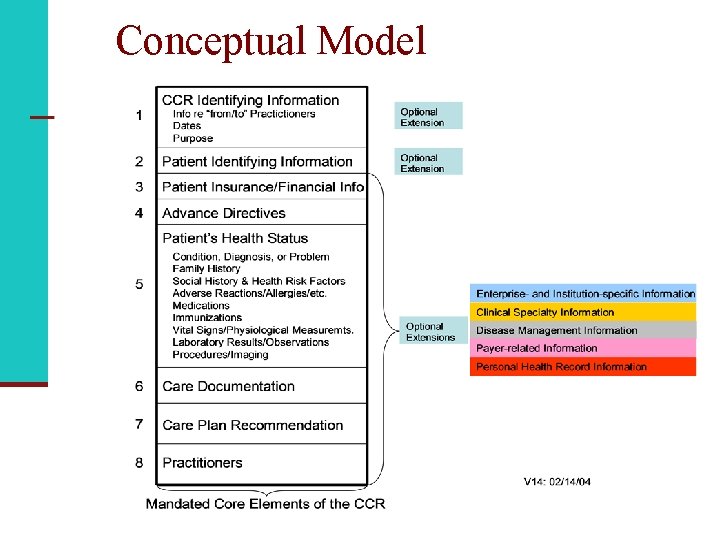
What’s in the CCR? n CCR identifying information n Patient insurance/financial information n Advance directives n Patient’s health status n Care documentation n Care plan recommendation n Practitioners

CCR Standard Specification n CCR scope n Referenced documents n Terminology (definitions) n Significance and Use n Specifications n Conceptual Model n Sections of CCR n Annex A: Spreadsheet of core elements n Annex B: XML schema n Annex C: Example report

Conceptual Model

Annex A of Standard Specification n Detailed list of the CCR data groups. n Fields n Associated definitions, comments, examples n Specification of whether field is required or optional n Required XML when preparing CCR in a structured electronic format n Notes n Date groups can be repeated as necessary n Wherever a code is used (e. g. , Diagnosis: 461. 9), the type and version of the system (e. g. , ICD-9 -CM) used to assign the code must be included. n Links where appropriate to Conditions/Diagnoses/Problems and Care Recommendations.

A Sample Data Element n Social History and Health Risk Factors n Definition: This Data Group provides information on social and personal factors that may impact the patient's health. n Comments/Examples: Smoking/Tobacco Use, Diet, Exercise, ETOH Use, Living Situation, Travel History, and Environmental or Occupational Exposures. n Required or Optional: Optional n XML: <RISK. FACTOR><ATTRIBUTE>

When Is the CCR Used? n Referral or transfer: Referring practitioner transmits the CCR to receiving practitioner and new care setting where patient is being sent so that it arrives before or with patient. n Discharge without a referral or transfer: CCR is provided to patient for future use, including visits to urgent care or emergency department, and to whomever patient designates as primary care practitioner responsible for followup care, if needed. n Personal health record: Patient keeps copies of his/her CCRs and supplements them, e. g. , with alternative medicine information and other PHI. n Other: Also useful to researchers and others not directly involved in patient’s treatment.

For Maximum Utility: XML n XML structured electronic format makes CCR n Interchangeable n Allows flexibility to prepare, transmit, and view CCR in multiple ways n n In a browser HL 7 CDA-compliant document Secure email Within any XML-enabled word processing document Allows display of fields in multiple formats n Allows interchange of CCR between otherwise incompatible EHR systems n

The EHR and the CCR n Using the XML specified in this standard, EHR systems will be able to import and export all CCR data n to enable automated healthcare information transmission with minimal workflow disruption for practitioners. n n The CCR will provide additional content and support for the EHR through extensions.

Extensions for Additional Content n Enterprise and institution-specific n Acute care, long-term care, home care, etc. n Clinical specialty-specific n Pediatrics, Nursing, etc. n Disease management n Disease-specific information, performance measures, guidelines, etc. n May be used by health plans, pharmas, patient advocacy groups, others promoting best practices n Payers n Additional financial information and care documentation n Patient-entered Personal Health Record n Complimentary and alternative medicine n Private or sensitive health information n Expanded family history

Other CCR-related Activities n HIMSS/HL 7 demonstration at HIMSS n Connectathon n CCR representatives assisting HL 7 with preparation n TEPR CCR demonstration n USB drive with CCR loaded on it n Will require secure access n Vendors will demonstrate ability to upload, read, and transmit CCR

Other CCR-related Activities n Potential for demonstration and implementation projects n Possible funding through private and public organizations, e. g. , AHRQ grants n Demonstration of utilization of CCR in movement of patients between practitioners and care-settings n Long-term care to/from acute care settings n Primary care to/from specialist n Acute care to/from home care n Several similar efforts internationally, e. g. , Finland, Denmark, England, The Netherlands, Germany, Spain

Development of CCR and Extensions n Meetings of stakeholders n Circulation and website postings of evolving standard n Balloting Requires ASTM membership n Nonmember database also developed for updates, meeting notices, opportunities for input n

CCR Timeline for 2004 -2005 n CCR balloting in February, results in March n April meeting agenda n Resolve negatives, if any n Expand awareness of CCR n Develop implementation guide n Develop extensions n Do demonstration projects n Ballot standards addressing extensions and implementation guide n Maintain/update standards

Thank you! n For more information on the CCR n Claudia Tessier, RHIA 202 -659 -2699 ctessi@attglobal. net