Statistics in Drug Development Rong Zhou Ph D























- Slides: 39

Statistics in Drug Development Rong Zhou, Ph. D Director, Biostatistics Medpace STATISTICS IN DRUG DEVELOPMENT 1

Topics q Introduction of pharmaceutical industry q Drug development process and clinical trials. q Biostatistics in clinical trials: ◦ Study design ◦ Clinical data ◦ Statistical modeling q Other topics STATISTICS IN DRUG DEVELOPMENT 2

Drug v Drug definition: according to the Food, Drug, and Cosmetic Act (FD&C Act): (1): a substance recognized in an official pharmacopoeia or formulary (2): a substance intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease (3): a substance other than food intended to affect the structure or function of the body (4): a substance intended for use as a component of a medicine but not a device or a component, part, or accessory of a device Novartis (60. 9 B), Simple version: A Chemical Substance that Interacts with a Living System and Produces a Biological Response STATISTICS IN DRUG DEVELOPMENT 3

Drug v“New Drug” vs “Generic Drug” Ø New Drug patents expire 20 years from the date of filing. Many other factors can affect the duration of a patent. Since the company applies for a patent long before the clinical trial to assess a drug’s safety and efficacy has commenced, the effective patent period after the drug has finally received approval is often around seven to twelve years. Ø New drugs (brand names) and generic drugs have the same pharmacological effects (dosage, intended use, effects, side effects, route of administration, risks, safety, etc. ) and pharmacokinetics. Ø For example: Glucophage vs. metformin. v“Prescription Only” vs “Over the Counter” v. Other drugs (orphan drugs), medical devices, vaccine STATISTICS IN DRUG DEVELOPMENT 4

Pharmaceutical Industry v List of 2013 top biotech and pharmaceutical companies (Revenue): Johnson & Jonson (71. 3 B), Novartis (60. 9 B), Roche (52. 4 B), Pfizer (51. 6 B), Sanofi, Merck, Glaxo. Smith. Kline, Astra. Zeneca, Eli Lilly, Abbott Lab. v. List of 2013 top biotech and pharmaceutical companies (Net income): Pfizer (US, 22 B), Johnson & Jonson (13. 8 B), Roche (Switzerland, 12. B), Novartis (Switzerland, 9. 5 B), Glaxo. Smith. Kline (UK, 9 B), Sanofi (France, 5. 1 B), Amgen (US, 5. 1 B), Eli Lilly, Novo Nordisk, Merck. STATISTICS IN DRUG DEVELOPMENT 5

Pharmaceutical Industry: Drug v Example of Blockbuster drugs from Astra. Zeneca: Astra. Zeneca's Nexium (heartburn and acid reflux medication) Sale: 5 B (2009), 5 B (2010), 4. 4 B (2011), 3. 9 B (2012), 6. 14 B (2013, the top 2 nd) Patent expired May 2014. Astra. Zeneca will not release an OTC drug. Astra. Zeneca's Crestor (indication: cholesterol) Sale: 5. 3 B (2013, the top 4 th). Patent is going to expire in 2016. v Example of Blockbuster drugs from Pfizer: Lipitor (Pfizer, indication: cholesterol) had sale Q 3/2012 as $0. 186 B. The patent expired in November, 2011. Lipitor sales in 2010: $10. 7 B for the year. STATISTICS IN DRUG DEVELOPMENT 6

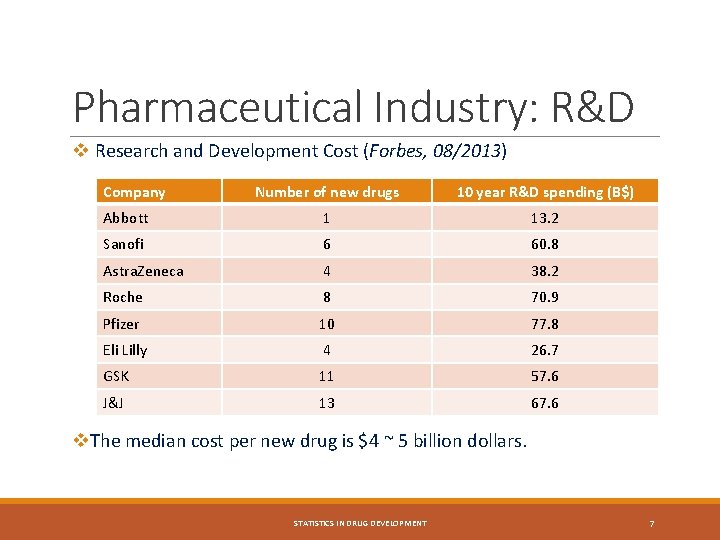
Pharmaceutical Industry: R&D v Research and Development Cost (Forbes, 08/2013) Company Number of new drugs 10 year R&D spending (B$) Abbott 1 13. 2 Sanofi 6 60. 8 Astra. Zeneca 4 38. 2 Roche 8 70. 9 Pfizer 10 77. 8 Eli Lilly 4 26. 7 GSK 11 57. 6 J&J 13 67. 6 v. The median cost per new drug is $4 ~ 5 billion dollars. STATISTICS IN DRUG DEVELOPMENT 7

Pharmaceutical Industry: CRO Contract Research Organization: A contract research organization (CRO) is an organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. CROs that specialize in clinical-trials services can offer their clients the expertise of moving a new drug or device from its conception to FDA/EMA marketing approval, without the drug sponsor having to maintain a staff for these services. Top CROs: Quintiles, PAREXEL, Covance, PPD, ICON The outsourcing market is about $20 -30 billions. Quintiles (Durham NC) has 30, 000+ employees in 60 countries. Year 2011 revenue is about $3. 00 billion, and Year 2013 revenue is $3. 8 billion. STATISTICS IN DRUG DEVELOPMENT 8

Agenda √ Introduction of pharmaceutical industry q Drug development process and clinical trials. q Biostatistics in clinical trials: ◦ Study design ◦ Clinical data ◦ Statistical modeling q Other topics STATISTICS IN DRUG DEVELOPMENT 9

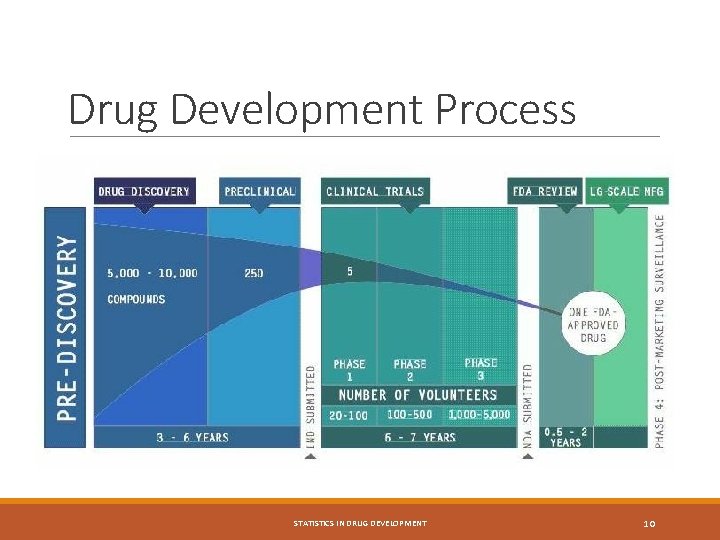
Drug Development Process STATISTICS IN DRUG DEVELOPMENT 10

Clinical Trials Visit Clinical. Trials. gov. STATISTICS IN DRUG DEVELOPMENT 11

Clinical Trial Phases Phase 1: Studies that are usually conducted with healthy volunteers and that emphasize safety. The goal is to find out what the drug's most frequent and serious adverse events are and, often, how the drug is metabolized and excreted (pharmacokinetics). ADME (Absorption, Distribution, Metabolism, and Excretion) will be studies. Phase 1 clinical trials include: § FIM (first-in-man) and/or single ascending dose cohorts study § Multiple ascending dose cohorts study § Food effect study § Drug-drug interaction study § Bioavailability and bioequivalence § PK study based on special populations § Thorough QT study STATISTICS IN DRUG DEVELOPMENT 12

Clinical Trial Phases Phase 2: Studies that gather preliminary data on effectiveness (whether the drug works in people who have a certain disease or condition). Safety continues to be evaluated, and short-term adverse events are studied. Phase 3: Studies that gather more information about safety and effectiveness by studying different populations and different dosages and by using the drug in comb Phase 4: Studies are done after the drug or treatment has been marketed to gather information on the drug's effect in various populations and any side effects associated with long-term use. STATISTICS IN DRUG DEVELOPMENT 13

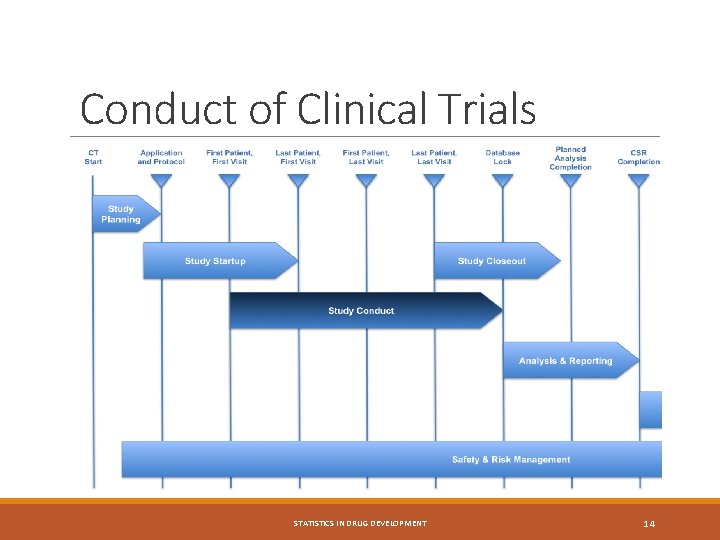
Conduct of Clinical Trials STATISTICS IN DRUG DEVELOPMENT 14

Agenda √ Introduction of pharmaceutical industry √ Drug development process and clinical trials. q Biostatistics in clinical trials: ◦ Study design ◦ Clinical data ◦ Statistical modeling q Other topics STATISTICS IN DRUG DEVELOPMENT 15

Biostatistics: Responsibilities Design the study and develop the protocol Design the randomization algorithm if needed Draft the statistical analysis plan (SAP) Programming the study data based on the SAP: tables/ figures/ listings (TFLs). Present the results and write the interpretation of study results in clinical study report (CSR). STATISTICS IN DRUG DEVELOPMENT 16

Biostatistics (1): Study Classification Phase I PK/PD study Thorough QT study Superiority study Active control and equivalence/non-inferiority study Dose-response study Safety follow-up study STATISTICS IN DRUG DEVELOPMENT 17

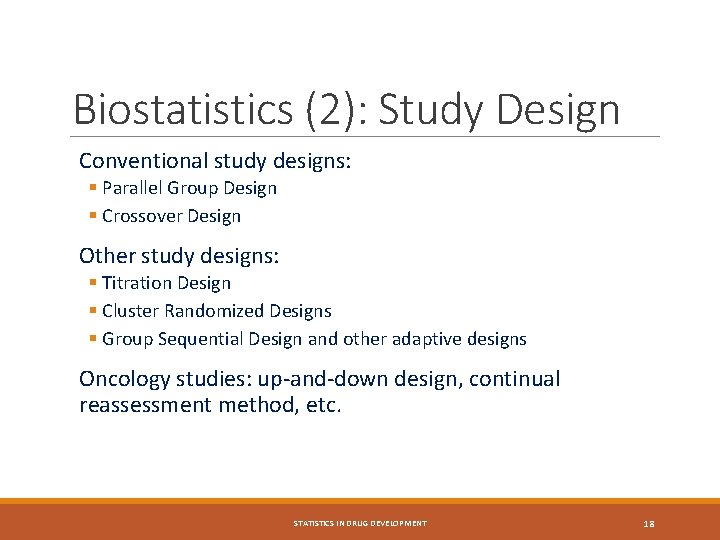
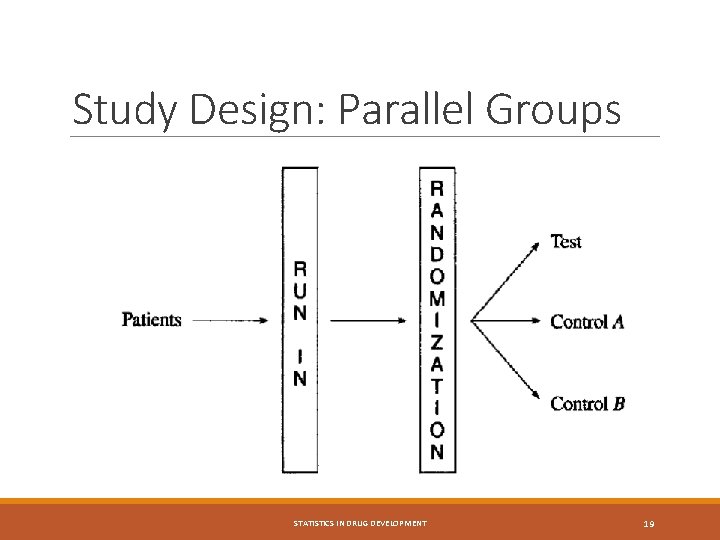
Biostatistics (2): Study Design Conventional study designs: § Parallel Group Design § Crossover Design Other study designs: § Titration Design § Cluster Randomized Designs § Group Sequential Design and other adaptive designs Oncology studies: up-and-down design, continual reassessment method, etc. STATISTICS IN DRUG DEVELOPMENT 18

Study Design: Parallel Groups STATISTICS IN DRUG DEVELOPMENT 19

Study Design: Cross-Over STATISTICS IN DRUG DEVELOPMENT 20

Things to Consider Selection of control: § Placebo control § Positive control Randomization § Is randomization necessary? § Cluster randomization? § Is randomization practical at certain level (such as site)? § Randomization algorithm: single site vs. multiple site, permutedblock method vs. dynamic method with minimization algorithm Blinding Sample size calculation STATISTICS IN DRUG DEVELOPMENT 21

Biostatistics (3): Clinical Data Continuous data § Lab measures & vital signs: blood pressure, blood glucose, etc. § Instruments: questionnaire scores, etc. § Calculated parameters: AUC, etc. Categorical data § Binary outcome: response (Yes or no), fatal, etc. § Multiple level outcome: event severity, number of events, etc. Censored data (time-to-event) § Time to disease progress § Time to heal Other data format, such as imaging data, questionnaires, ROC STATISTICS IN DRUG DEVELOPMENT 22

Biostatistics (4): Case Studies Case Study 1: Diabetes Case Study 2: Stroke Response Case Study 3: Cardiovascular Endpoint Study Case Study 4: Pharmacokinetics Study STATISTICS IN DRUG DEVELOPMENT 23

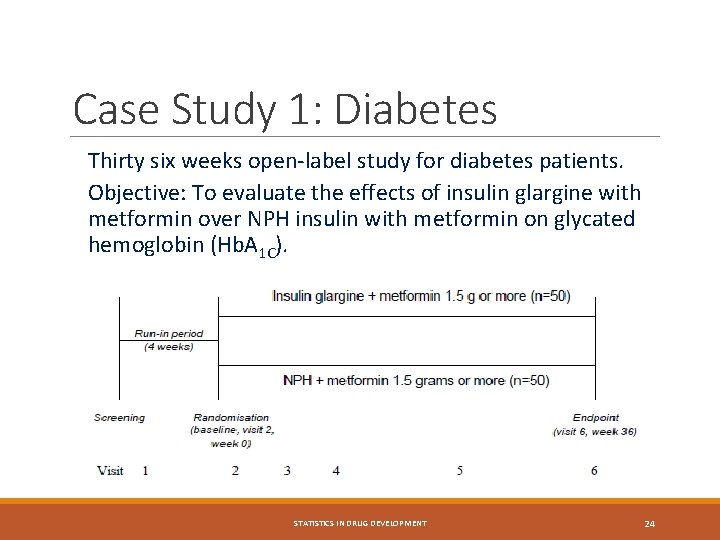
Case Study 1: Diabetes Thirty six weeks open-label study for diabetes patients. Objective: To evaluate the effects of insulin glargine with metformin over NPH insulin with metformin on glycated hemoglobin (Hb. A 1 C). STATISTICS IN DRUG DEVELOPMENT 24

Case Study 1 The primary efficacy variable is the change in Hb. A 1 c value from baseline (0 weeks) to last visit (36 weeks). The primary analysis will be performed using an analysis of covariance (ANCOVA) model with Hb. A 1 c change from baseline as a response variable, treatment group and center as fixed effects, and the baseline value as a covariate. STATISTICS IN DRUG DEVELOPMENT 25

Case Study 1 How to define the endpoint: § End of study (EOS) measures (Week 36): what if missing, what if off drug for more than two weeks? What if… § Change from baseline to EOS § Percent change from baseline to EOS § Two data points vs. one to reduce variability? § Log transformation or other transformations: still make senses? Statistical modeling: § Modeling diagnosis § Need to add more factors/predictors or remove some: country, sex, race, etc. ? § Non-parametric approach? STATISTICS IN DRUG DEVELOPMENT 26

Case Study 2: Stroke § This is a Phase 2, randomized, double-blind, placebo-controlled, multicenter study. The total trial duration is 12 months. § Approximately 200 subjects who experienced stroke will be randomized (100: 100) into the trial. § The modified Rankin Scale (m. RS) score will be assessed at Day 90 and used as efficacy. The m. RS is a commonly used scale for measuring the degree of disability or dependence in the daily activities of people who have suffered a stroke or other causes of neurological disability, and it has become the most widely used clinical outcome measure for stroke clinical trials. § The m. RS score runs from 0 -6, running from perfect health without symptoms to death. 0 - No symptoms. 1 - No significant disability. 2 Slight disability. 3 - Moderate disability. 4 - Moderately severe disability. 5 - Severe disability. 6 - Dead. STATISTICS IN DRUG DEVELOPMENT 27

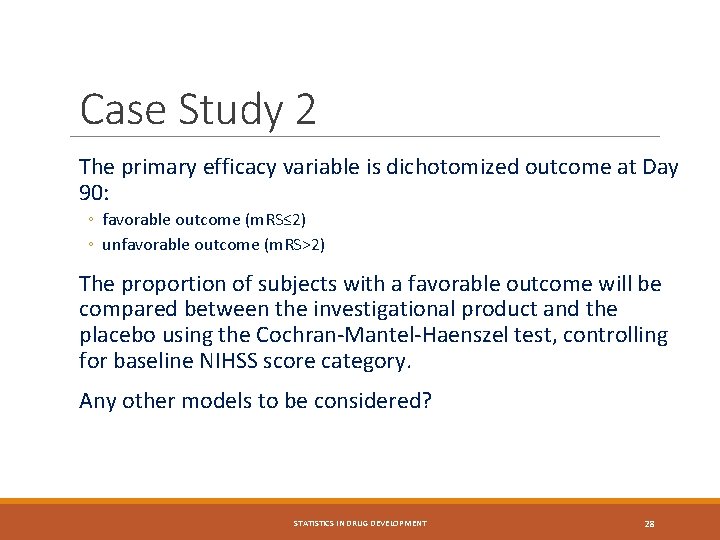
Case Study 2 The primary efficacy variable is dichotomized outcome at Day 90: ◦ favorable outcome (m. RS≤ 2) ◦ unfavorable outcome (m. RS>2) The proportion of subjects with a favorable outcome will be compared between the investigational product and the placebo using the Cochran-Mantel-Haenszel test, controlling for baseline NIHSS score category. Any other models to be considered? STATISTICS IN DRUG DEVELOPMENT 28

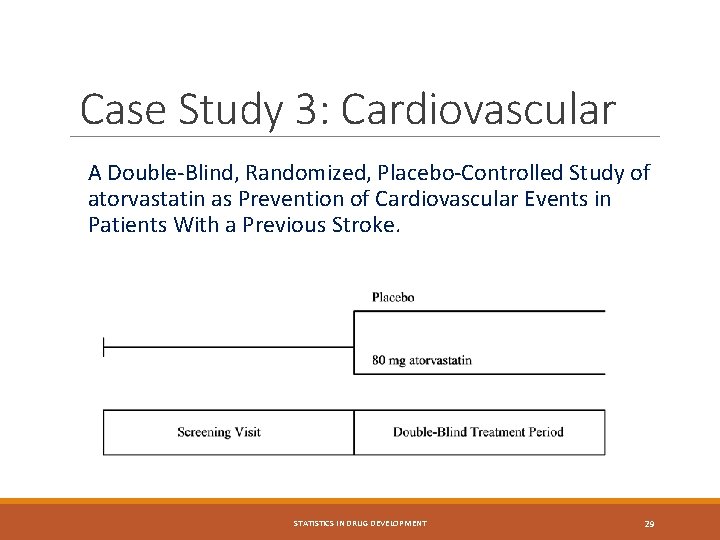
Case Study 3: Cardiovascular A Double-Blind, Randomized, Placebo-Controlled Study of atorvastatin as Prevention of Cardiovascular Events in Patients With a Previous Stroke. STATISTICS IN DRUG DEVELOPMENT 29

Case Study 3 The primary efficacy variable is the time from randomization to the first occurrence of a primary clinical endpoint (fatal or nonfatal stroke) A Cox proportional hazards regression analysis will be performed on the time from randomization to the first occurrence of a primary clinical endpoint. The primary model will contain the following covariates: treatment, center, and entry event (stroke). SAS procedure PROC PHREG will be used. Kaplan–Meier estimator (the product limit estimator) of the survival function will be plotted. STATISTICS IN DRUG DEVELOPMENT 30

Case Study 3 Data Handling: § Data from all randomized patients will be analyzed. § Patients who experience a primary clinical endpoint are considered as completer. § Censoring occurs for patients who do not experience a primary clinical endpoint prior to the completion of the study. The censoring time will correspond to the study day on which the patient completed the study or was last contacted during the study following a withdrawal. § If a patient dies from a cause other than stroke, the survival time will be censored as if the patient had been lost to followup at that point. STATISTICS IN DRUG DEVELOPMENT 31

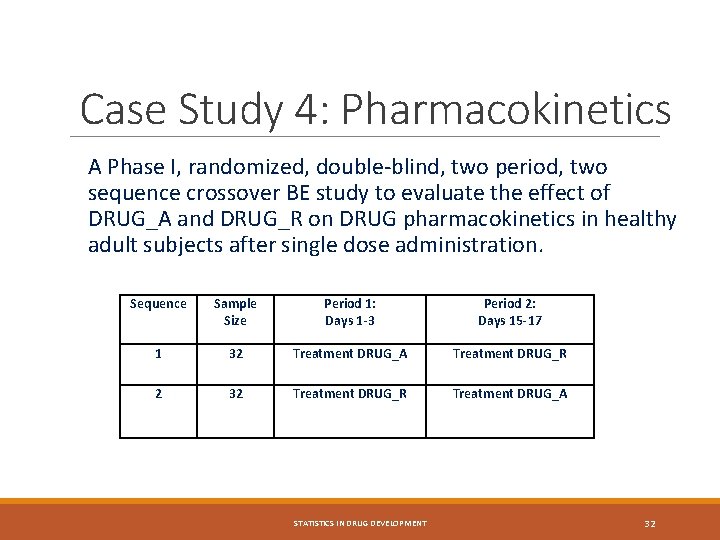
Case Study 4: Pharmacokinetics A Phase I, randomized, double-blind, two period, two sequence crossover BE study to evaluate the effect of DRUG_A and DRUG_R on DRUG pharmacokinetics in healthy adult subjects after single dose administration. Sequence Sample Size Period 1: Days 1 -3 Period 2: Days 15 -17 1 32 Treatment DRUG_A Treatment DRUG_R 2 32 Treatment DRUG_R Treatment DRUG_A STATISTICS IN DRUG DEVELOPMENT 32

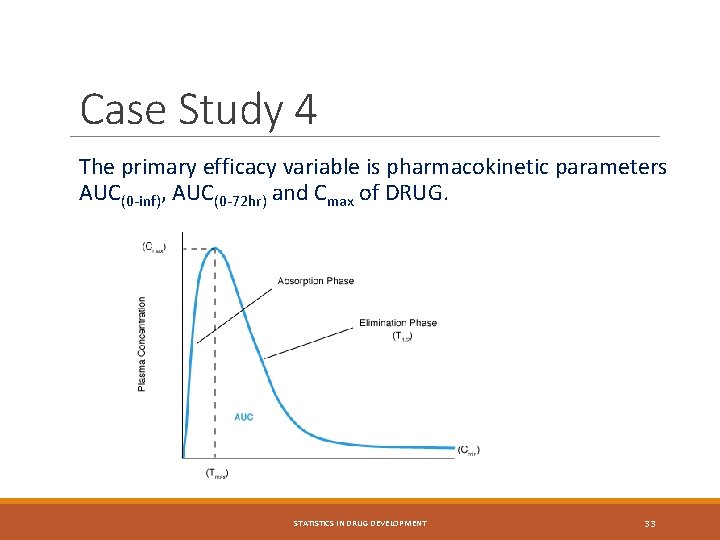
Case Study 4 The primary efficacy variable is pharmacokinetic parameters AUC(0 -inf), AUC(0 -72 hr) and Cmax of DRUG. STATISTICS IN DRUG DEVELOPMENT 33

Case Study 4 The statistical analysis will be performed on the logtransformed pharmacokinetic parameters. Analysis of variance will be performed using SAS Mixed Linear Models procedure. Subject will be fitted as a random effect and treatment, sequence, period will be fitted as fixed effects in the model. The ratio and associated 90% CI will be estimated for the PK parameters. An example of SAS code is included here. Proc Mixed; class subject treatment; model log. PKvar = treatment seq period; random subject; lsmeans treatment; estimate 'test vs ref' treatment -1 1/cl alpha=0. 1; run; STATISTICS IN DRUG DEVELOPMENT 34

Agenda √ Introduction of pharmaceutical industry √ Drug development process and clinical trials. √ Biostatistics in clinical trials: ◦ Study design ◦ Clinical data ◦ Statistical modeling q Other topics STATISTICS IN DRUG DEVELOPMENT 35

Oncology Studies National Cancer Institute at NIH: The recent breakthroughs in understanding the molecular biology of cancer have led to an unprecedented number of new targets in oncology drug development. There are more cancer drugs in the research pipeline than any other type of therapy, corresponding directly with the number of oncology clinical trials. Different study designs and data analysis methods for oncology studies than for other drugs. STATISTICS IN DRUG DEVELOPMENT 36

Current Stat Research Topics v Adaptive design v Missing data imputation v Dose finding by the Continual Reassessment Method (CRM) v Randomization algorithm and randomization tests v Risk-based monitoring and Fraud Detection v. Design and analysis of non-inferiority studies STATISTICS IN DRUG DEVELOPMENT 37

Medpace Biostatistics Departments and Groups: § Biostatistician § Statistical analyst § Data manager, data coordinator § Clinical database programmer, SAS programmer § IVRS manager, coordinator § ECG core lab, imaging core lab Biostatistics Department: § Around 40 biostatisticians and statistical analysts § Two in Scotland, UK, and the rest in Cincinnati office § SAS is the primary software. Currently using Version 9. 3 STATISTICS IN DRUG DEVELOPMENT 38

Q&A STATISTICS IN DRUG DEVELOPMENT 39