Statistics for Microarrays Biological background Molecular Laboratory Techniques
Statistics for Microarrays Biological background: Molecular Laboratory Techniques Class web site: http: //statwww. epfl. ch/davison/teaching/Microarrays/ETHZ/

Molecular Laboratory Techniques • Hybridizing DNA • Copying DNA • Cutting DNA • Probing DNA
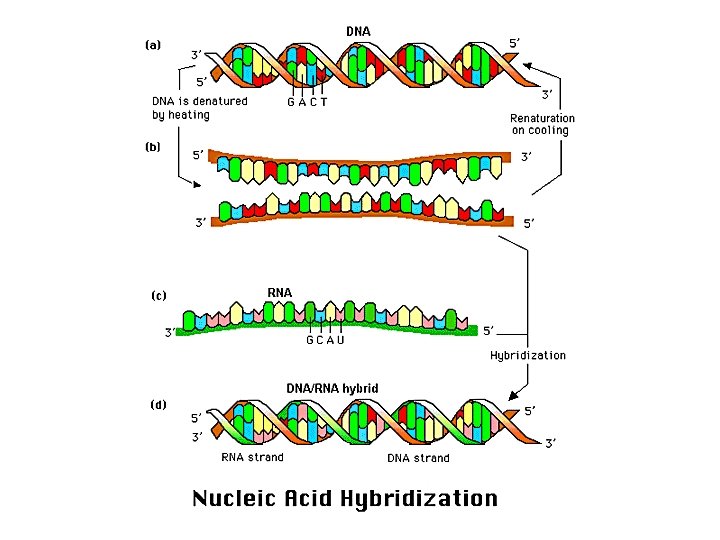
Hybridization • Hybridization exploits a potent feature of the DNA duplex – the sequence complementarity of the two strands • Strands can be separated (denatured) by heating • Remarkably, DNA can reassemble with perfect fidelity from separated strands
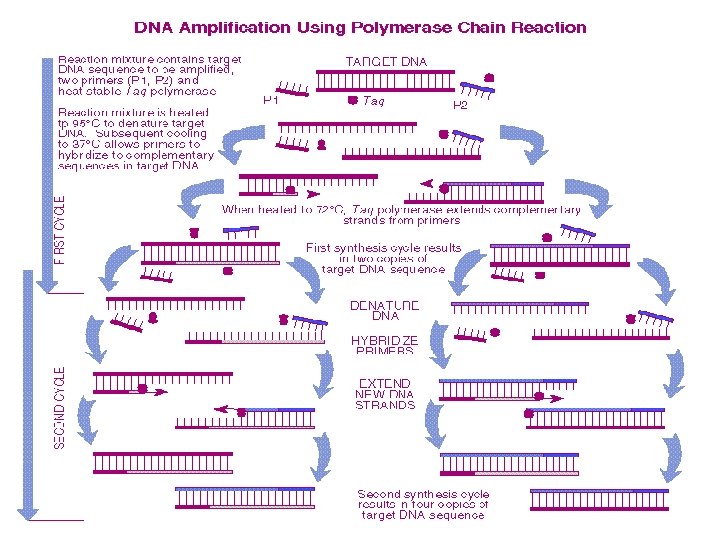
Polymerase Chain Reaction (PCR) • PCR is used to amplify (copy) specific DNA sequences in a complex mixture when the ends of the sequence are known • Source DNA is denatured into single strands • Two synthetic oligonucleotides complementary to the 3’ ends of the segment of interest are added in great excess to the denatured DNA, then the temperature is lowered • The genomic DNA remains denatured since the complementary strands are at too low a concentration to encounter each other during the period of incubation • The specific oligonucleotides hybridize with complementary sequences in the genomic DNA
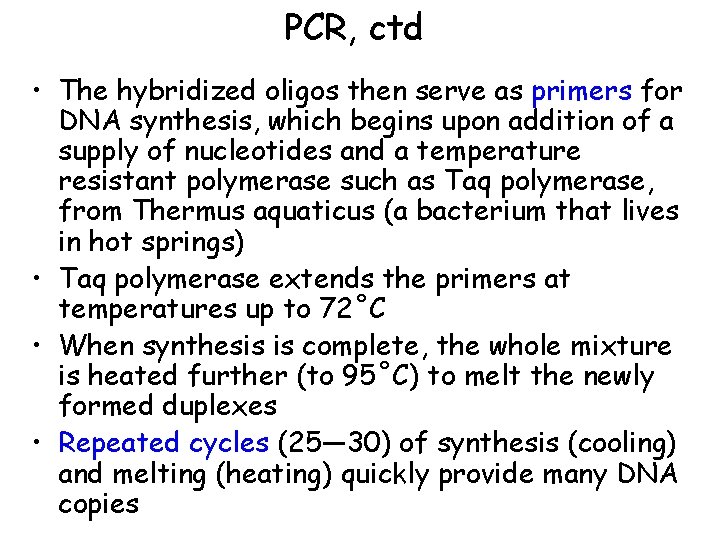
PCR, ctd • The hybridized oligos then serve as primers for DNA synthesis, which begins upon addition of a supply of nucleotides and a temperature resistant polymerase such as Taq polymerase, from Thermus aquaticus (a bacterium that lives in hot springs) • Taq polymerase extends the primers at temperatures up to 72˚C • When synthesis is complete, the whole mixture is heated further (to 95˚C) to melt the newly formed duplexes • Repeated cycles (25— 30) of synthesis (cooling) and melting (heating) quickly provide many DNA copies
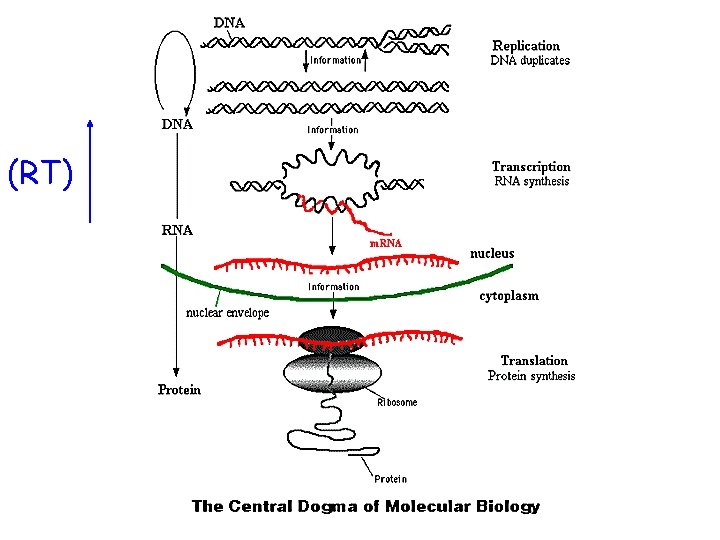
(RT)
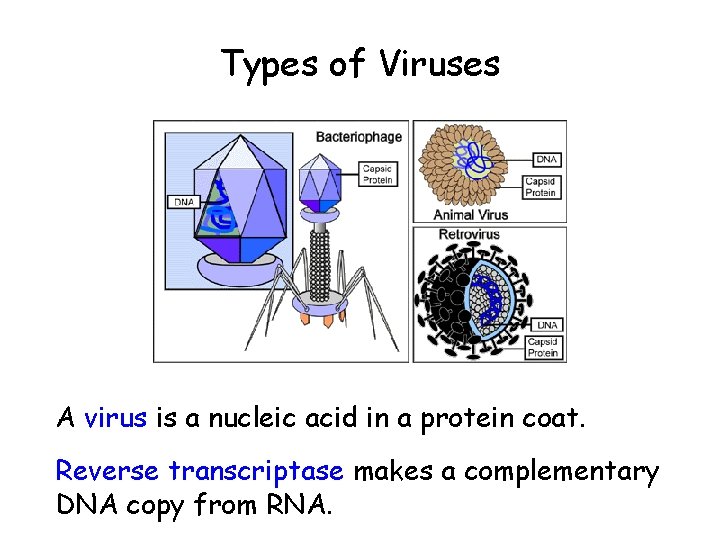
Types of Viruses A virus is a nucleic acid in a protein coat. Reverse transcriptase makes a complementary DNA copy from RNA.

Reverse transcription Clone c. DNA strands, complementary to the m. RNA GUAAUCCUC Reverse transcriptase c. DNA TT AG GA G CA TTA AG GG G A GA G C ACTATTAAG CA TTAT GTG GAG A G CT A TTA G CC G GG AAGG T A G A T T A G CATTAGGAG CA
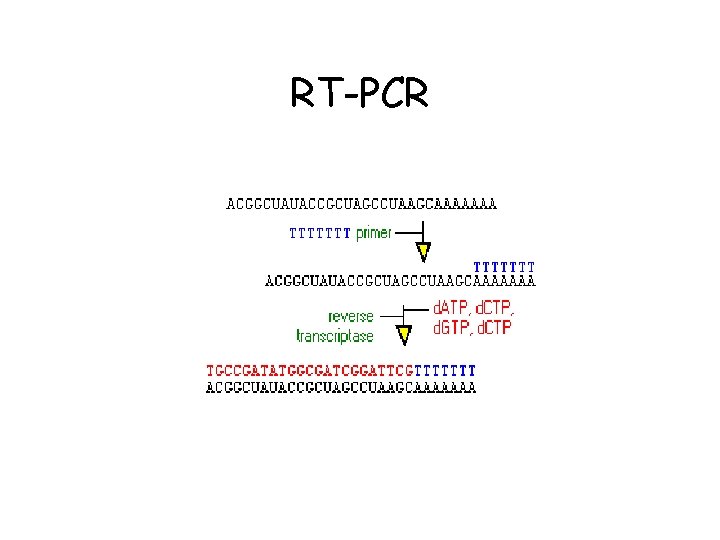
RT-PCR
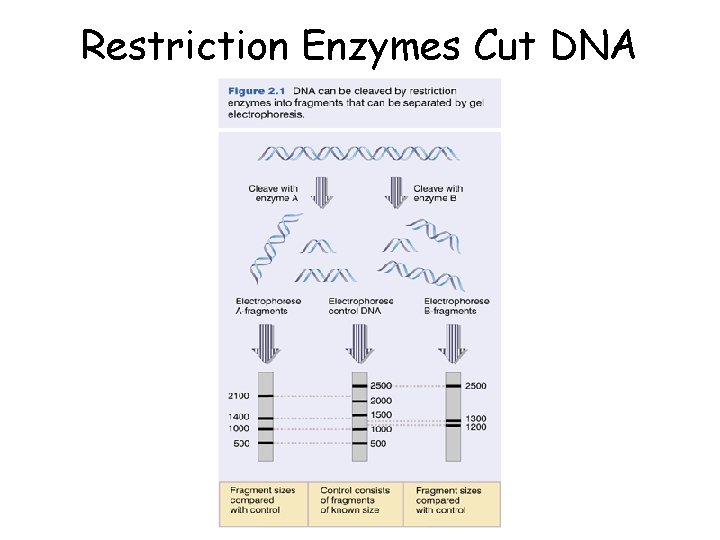
Restriction Enzymes Cut DNA
Probing DNA • One way to study a specific DNA fragment within a genome is to probe for the sequence of the fragment • A probe is a labeled (usually radioactive or fluorescent) single-stranded oligonucleotide, synthesized to be complementary to the sequence of interest – probe sequence is known • Attach single-stranded DNA to a membrane (or other solid support) and incubate with the probe so that it hybridizes • Visualize the probe (e. g. by X-ray for radioactive probes)
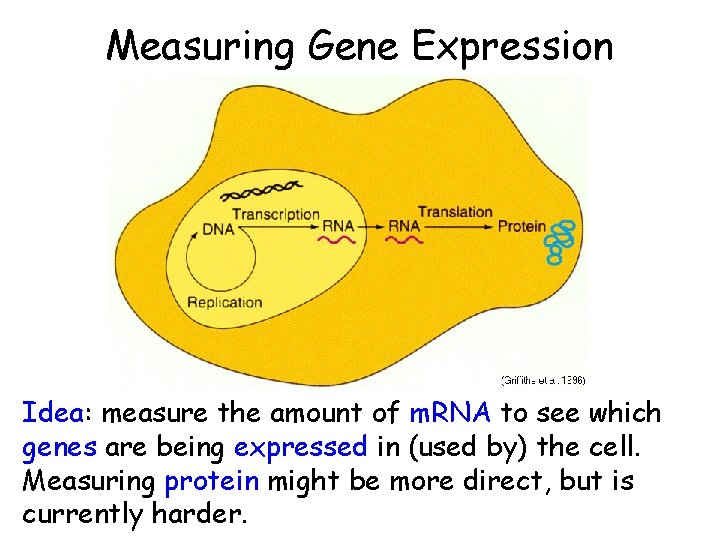
Measuring Gene Expression Idea: measure the amount of m. RNA to see which genes are being expressed in (used by) the cell. Measuring protein might be more direct, but is currently harder.
Principal Uses of Microarrays • Genome-scale gene expression analysis – Differential gene expression between two (or more) sample types – Responses to environmental factors – Disease processes (e. g. cancer) – Effects of drugs – Identification of genes associated with clinical outcomes (e. g. survival) • Detection of sequence variation – Genetic typing – Detection of somatic mutations (e. g. in oncogenes) – Direct sequencing
Major technologies • c. DNA probes (> 200 nt), usually produced by PCR, attached to either nylon or glass supports • Oligonucleotides (25 -80 nt) attached to glass support • Oligonucleotides (25 -30 nt) synthesized in situ on silica wafers (Affymetrix) • Probes attached to tagged beads
Brief outline of steps for producing a c. DNA microarray • Probes are c. DNA fragments, usually amplified by PCR • Probes are deposited on a solid support, either positively charged nylon or glass slide • Samples (normally poly(A)+ RNA) are labelled using fluorescent dyes • At least two samples are hybridized to chip • Fluorescence at different wavelengths measured by a scanner
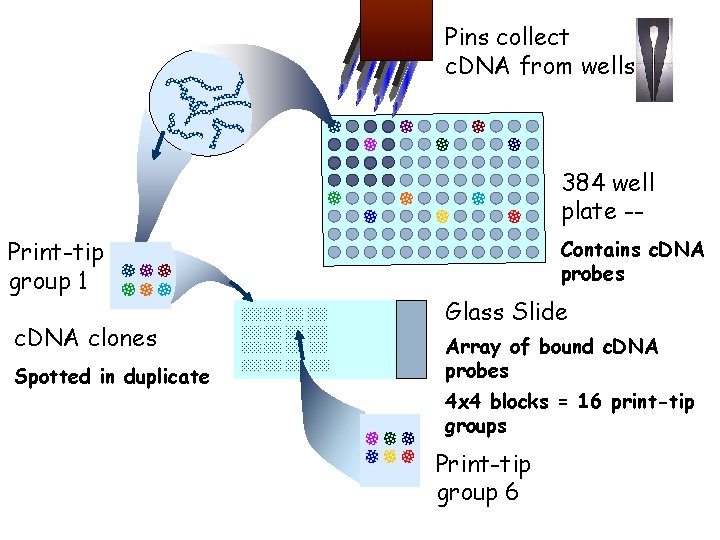
Pins collect c. DNA from wells 384 well plate -Print-tip group 1 c. DNA clones Spotted in duplicate Contains c. DNA probes Glass Slide Array of bound c. DNA probes 4 x 4 blocks = 16 print-tip groups Print-tip group 6
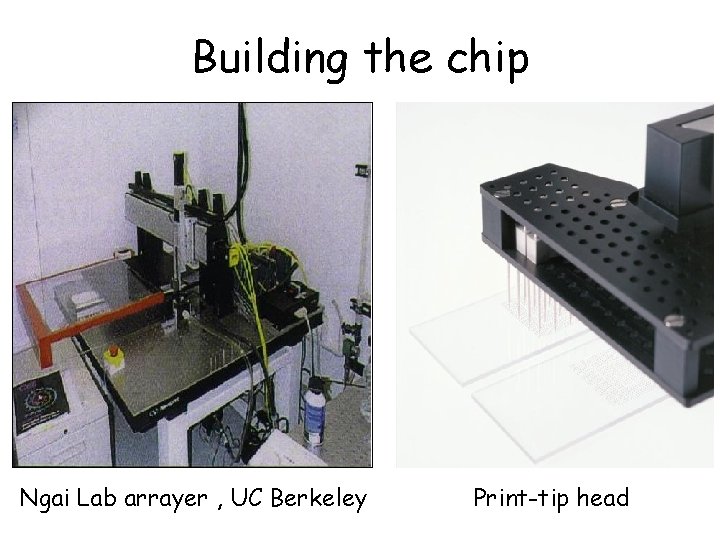
Building the chip Ngai Lab arrayer , UC Berkeley Print-tip head
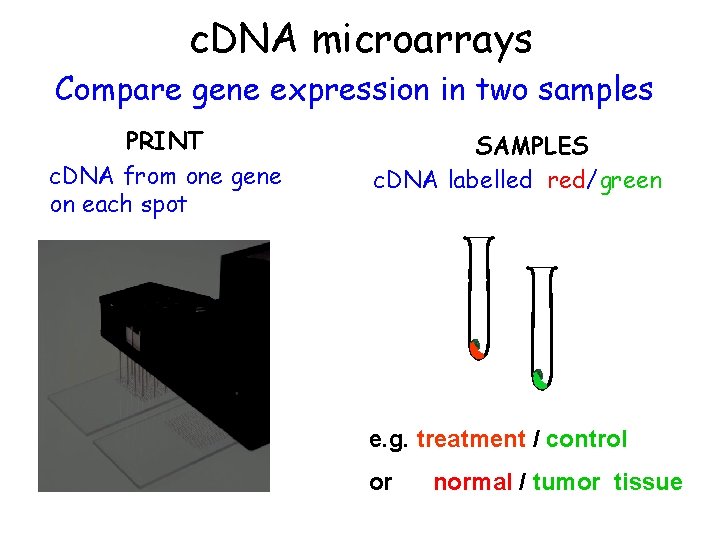
c. DNA microarrays Compare gene expression in two samples PRINT c. DNA from one gene on each spot SAMPLES c. DNA labelled red/green e. g. treatment / control or normal / tumor tissue
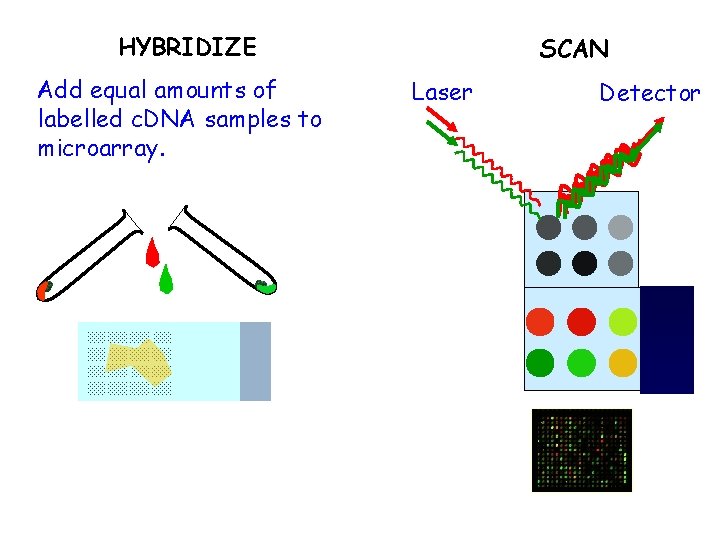
HYBRIDIZE Add equal amounts of labelled c. DNA samples to microarray. SCAN Laser Detector
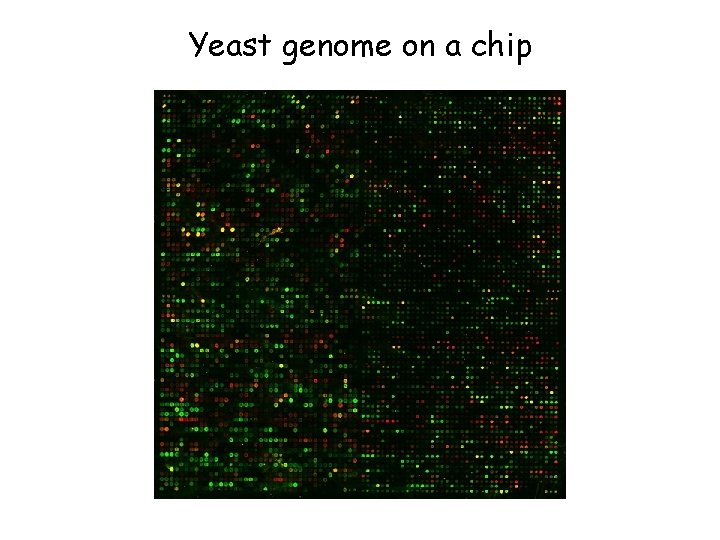
Yeast genome on a chip

Web animation of a c. DNA microarray experiment http: //www. bio. davidson. edu/courses/genomics/chip/ chip. html
c. DNA Microarray Design • Probe selection – Non-redundant set of probes – Includes genes of interest to project – Corresponds to physically available clones • Chip layout – Grouping of probes by function – Correspondence between wells in microtiter plates and spots on the chip

c. DNA arrays on nylon and glass • Nylon arrays – Up to about 1000 probes per filter – Use radiolabeled c. DNA target – Can use phosphorimager or X-ray film • Glass arrays – Up to about 40, 000 probes per slide, or 10, 000 per 2 cm 2 area (limited by arrayer’s capabilities) – Use fluorescent targets – Require specialized scanner
Glass chip manufacturing • Choice of coupling method – Physical (charge), non-specific chemical, specific chemical (modified PCR primer) • Choice of printing method – Mechanical pins: flat tip, split tip, pin & ring – Piezoelectric deposition (“ink-jet”) • Robot design – Precision of movement in 3 axes – Speed and throughput – Number of pins, numbers of spots per pin load
Scanning the arrays • Laser scanners – Excellent spatial resolution – Good sensitivity, but can bleach fluorochromes – Still rather slow • CCD scanners – Spatial resolution can be a problem – Sensitivity easily adjustable (exposure time) – Faster and cheaper than lasers • In all cases, raw data are images showing fluorescence on surface of chip
Affymetrix Gene. Chips • Probes are oligos synthesized in situ using a photolithographic approach • There at least 5 oligos per c. DNA, plus an equal number of negative controls • The apparatus requires a fluidics station for hybridization and a special scanner • Only a single fluorochrome is used per hybridization • Expensive, but getting cheaper

Affymetrix chip production
Commercial chips • Clontech, Incyte, Research Genetics filter-based arrays with up to about 8000 clones • Incyte / Synteni – 10, 000 probe chips, not distributed (have to send them target RNA) • Affymetrix - oligo-based chips with 12, 000 genes of known function (16 oligos/gene) and 4 x 10’ 000 genes from ESTs
Alternative technologies • Synthesis of probes on microbeads – Hybridization in solution – Identification of beads by fluorescent bar coding by embedding transponders – Readout using micro-flow cells or optic fiber arrays • Production of “universal” arrays – Array uses a unique combination of oligos, and probes containing the proper complements
c. DNA microarray experiments m. RNA levels compared in many different contexts • Different tissues, same organism (brain v. liver) • Same tissue, same organism (ttt v. ctl, tumor v. non-tumor) • Same tissue, different organisms (wt v. ko, tg, or mutant) • Time course experiments (effect of ttt, development) • Other special designs (e. g. to detect spatial patterns)
Arrays for Genetic Analysis • Mutation detection – Oligos (Affymetrix type) representing all known alleles – PCR followed by primer extension, with detection of alleles by MALDI-TOF mass spectroscopy (Sequenom) • Gene loss and amplification – Measure gene dosage in genomic DNA by hybridization to genomic probes
Microarray data on the Web • Many groups have made their raw data available, but in many formats • Some groups have created searchable databases • Several initiatives to create “unified” databases – EBI: Array. Express – NCBI: Gene Expression Omnibus • Some companies are beginning to sell microarray expression data (e. g. Incyte)
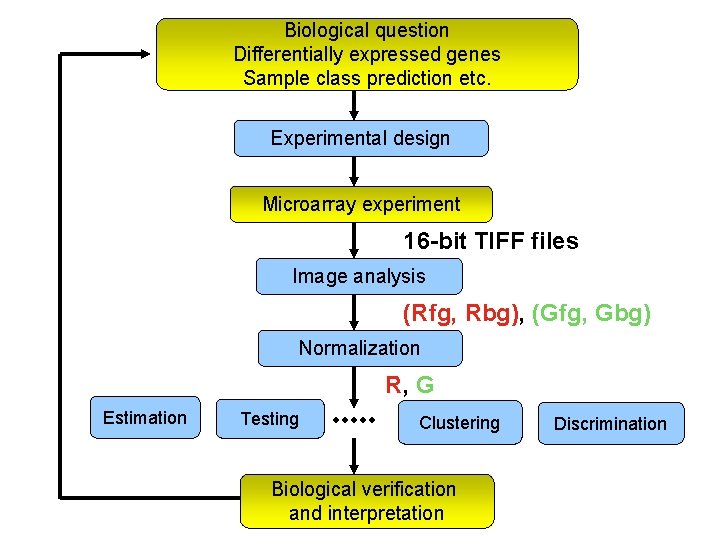
Biological question Differentially expressed genes Sample class prediction etc. Experimental design Microarray experiment 16 -bit TIFF files Image analysis (Rfg, Rbg), (Gfg, Gbg) Normalization R, G Estimation Testing Clustering Biological verification and interpretation Discrimination
- Slides: 35