Statistical Methods for Biotechnology Products II Design and

Statistical Methods for Biotechnology Products II Design and Classification of Clinical Trials Instructor: Jen-pei Liu, Ph. D. Division of Biometry Department of Agronomy National Taiwan University, and Division of Biostatistics and Bioinformatics National Health Research Institutes 1/1/2022 Copyright by Jen-pei Liu, Ph. D 1
Statistical Designs n Parallel Group Designs The patients are randomized to one of two or more groups, each group being allocated to a different treatment. n n Advantages n Simple and easy to implement. n Less complicated analysis and interpretation. Drawbacks n Relative large variability Inter-patient + Intra-patient 1/1/2022 Copyright by Jen-pei Liu, Ph. D 2
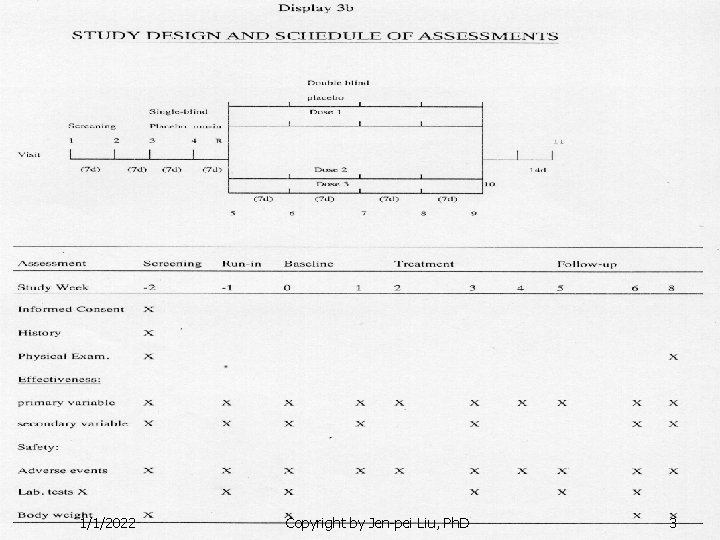
1/1/2022 Copyright by Jen-pei Liu, Ph. D 3
Parallel Group Design n A parallel group design is a completely randomized design (CRD) n n Group comparison designs Matched pairs parallel design n 1/1/2022 Each patient within the pairs is matched with some important prognostic factors One patient in the pair is assigned to the test drug and other patient is assigned to placebo Difficult to implement if # of factors is large and rarely utilize in practice Copyright by Jen-pei Liu, Ph. D 4
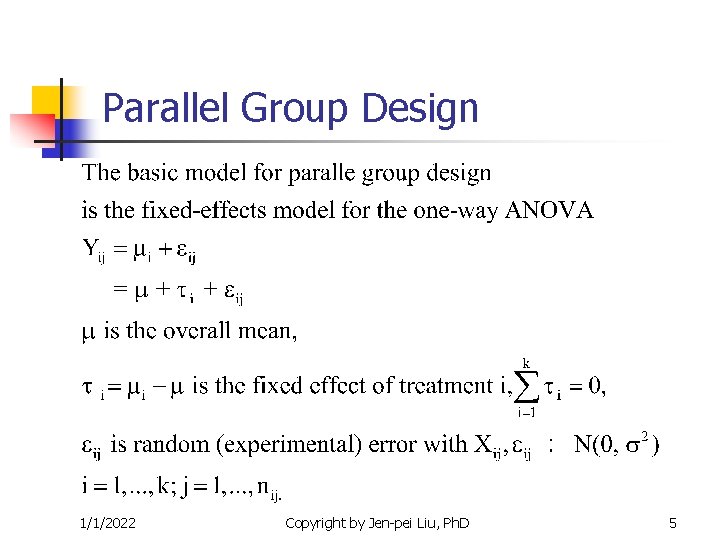
Parallel Group Design 1/1/2022 Copyright by Jen-pei Liu, Ph. D 5

Parallel Group Design n Run-in period n A period of placebo, no active treatment, dietary control, or active maintenance therapy prior to randomization n n 1/1/2022 A washout period to remove effects of previous therapy A period of obtaining baseline, a training period for patients, investigator and staff A period of identifying placebo responders and noncompliant To estimate placebo effect between the groups Copyright by Jen-pei Liu, Ph. D 6
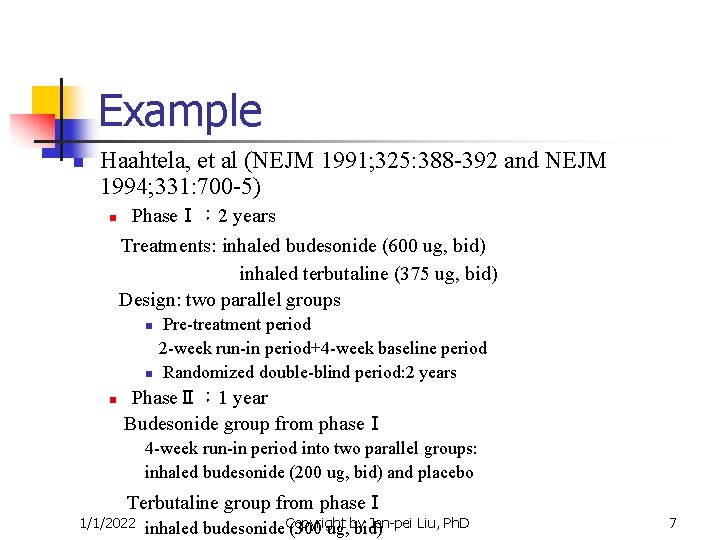
Example n Haahtela, et al (NEJM 1991; 325: 388 -392 and NEJM 1994; 331: 700 -5) n PhaseⅠ: 2 years Treatments: inhaled budesonide (600 ug, bid) inhaled terbutaline (375 ug, bid) Design: two parallel groups n n n Pre-treatment period 2 -week run-in period+4 -week baseline period Randomized double-blind period: 2 years PhaseⅡ: 1 year Budesonide group from phaseⅠ 4 -week run-in period into two parallel groups: inhaled budesonide (200 ug, bid) and placebo Terbutaline group from phaseⅠ 1/1/2022 Jen-pei Liu, Ph. D inhaled budesonide Copyright (300 ug, by bid) 7
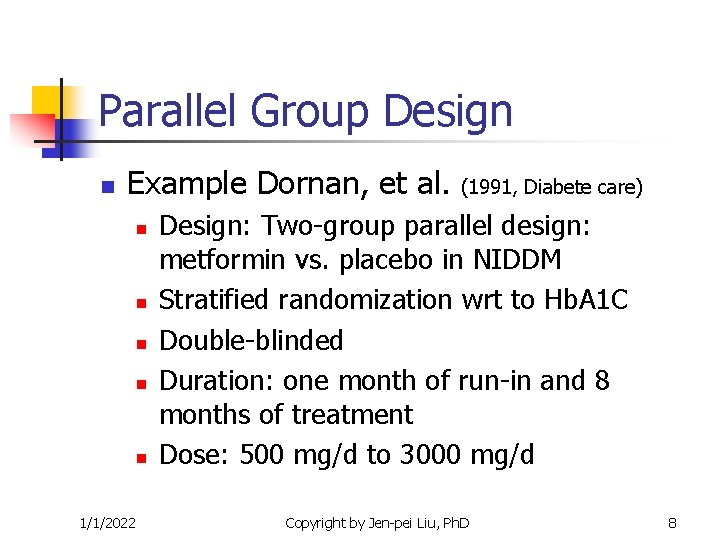
Parallel Group Design n Example Dornan, et al. n n n 1/1/2022 (1991, Diabete care) Design: Two-group parallel design: metformin vs. placebo in NIDDM Stratified randomization wrt to Hb. A 1 C Double-blinded Duration: one month of run-in and 8 months of treatment Dose: 500 mg/d to 3000 mg/d Copyright by Jen-pei Liu, Ph. D 8
Crossover Design Each subject is randomized to a sequence of two or more treatments Each subject receives two or more treatments in a study n Advantages n n n 1/1/2022 Subjects act their own control Reduction of variability Fewer patients required Copyright by Jen-pei Liu, Ph. D 9

Crossover Design n Drawbacks More difficult to implement n For stable and chronic diseases only n Biased inference due to carryover effects n More complicated analysis and interpretation n 1/1/2022 Copyright by Jen-pei Liu, Ph. D 10
1/1/2022 Copyright by Jen-pei Liu, Ph. D 11
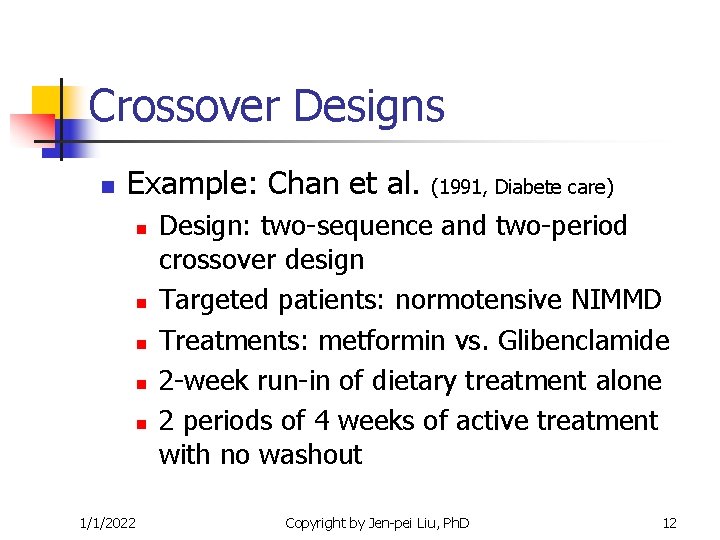
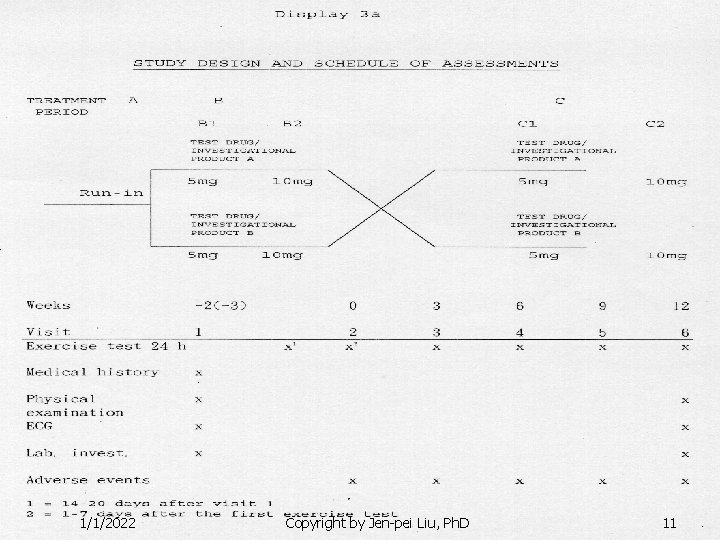
Crossover Designs n Example: Chan et al. n n n 1/1/2022 (1991, Diabete care) Design: two-sequence and two-period crossover design Targeted patients: normotensive NIMMD Treatments: metformin vs. Glibenclamide 2 -week run-in of dietary treatment alone 2 periods of 4 weeks of active treatment with no washout Copyright by Jen-pei Liu, Ph. D 12
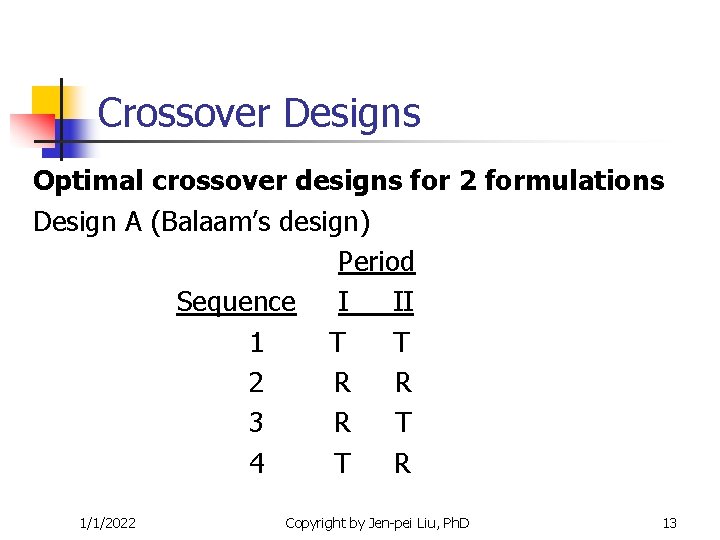
Crossover Designs Optimal crossover designs for 2 formulations Design A (Balaam’s design) Period Sequence I II 1 T T 2 R R 3 R T 4 T R 1/1/2022 Copyright by Jen-pei Liu, Ph. D 13
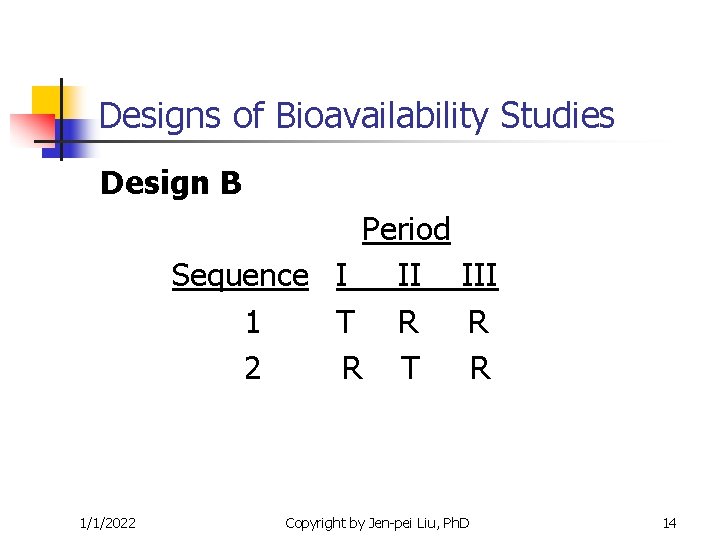
Designs of Bioavailability Studies Design B Period Sequence I II III 1 T R R 2 R T R 1/1/2022 Copyright by Jen-pei Liu, Ph. D 14
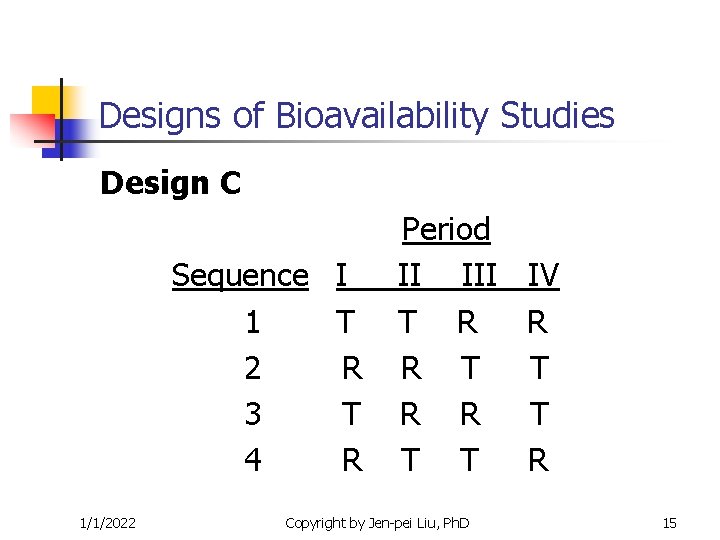
Designs of Bioavailability Studies Design C Sequence I 1 T 2 R 3 T 4 R 1/1/2022 Period II III T R R T T Copyright by Jen-pei Liu, Ph. D IV R T T R 15

Crossover Designs Complete crossover design: each sequence contains all formulations A g x p crossover design: There are g sequences of formulations administered at p different time periods. Washout period: Rest period between two treatment periods for which the effect of one formulation administered at one treatment period does not carry over to the next. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 16

Crossover Designs Direct drug effect: The effect that a drug product has during the period in which the drug is administered. Carryover effect: The effect that persists after the end of the dosing period. C-order carryover effect: The carryover effects last up to c treatment periods. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 17
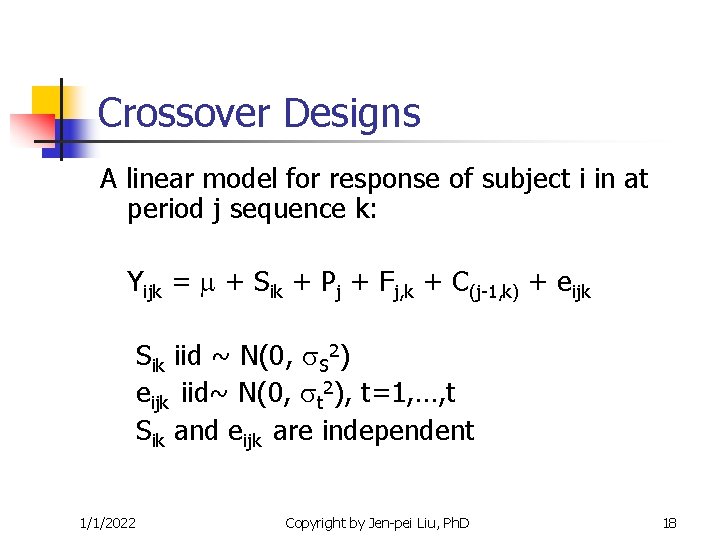
Crossover Designs A linear model for response of subject i in at period j sequence k: Yijk = + Sik + Pj + Fj, k + C(j-1, k) + eijk Sik iid ~ N(0, S 2) eijk iid~ N(0, t 2), t=1, …, t Sik and eijk are independent 1/1/2022 Copyright by Jen-pei Liu, Ph. D 18
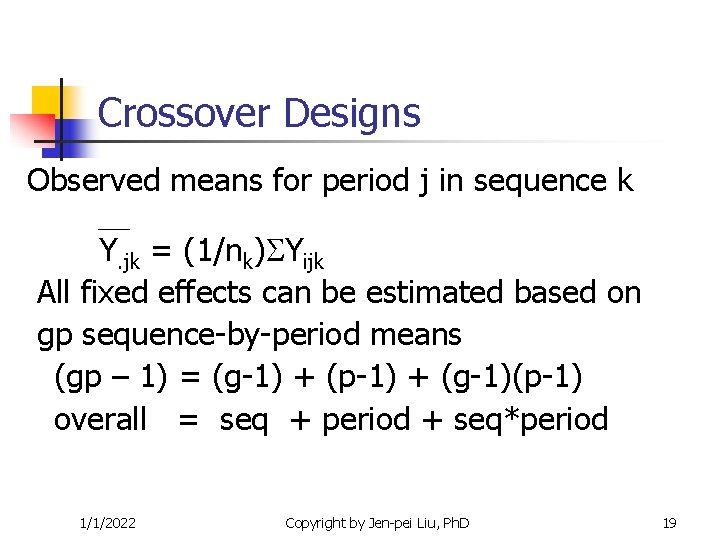
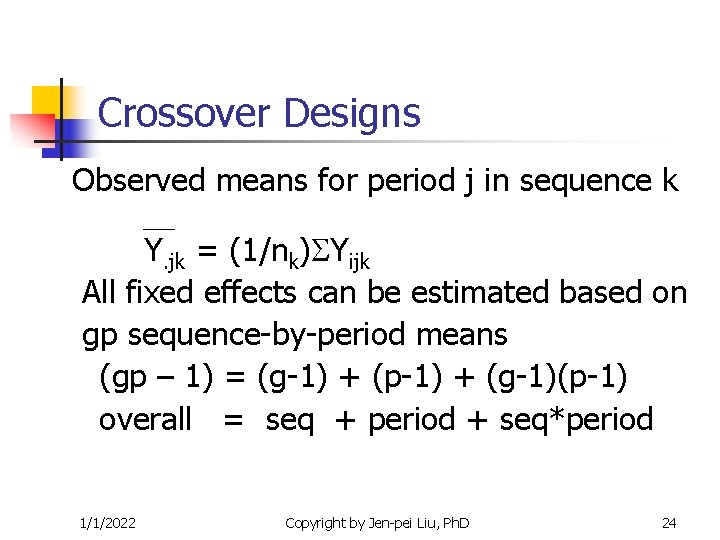
Crossover Designs Observed means for period j in sequence k Y. jk = (1/nk) Yijk All fixed effects can be estimated based on gp sequence-by-period means (gp – 1) = (g-1) + (p-1) + (g-1)(p-1) overall = seq + period + seq*period 1/1/2022 Copyright by Jen-pei Liu, Ph. D 19
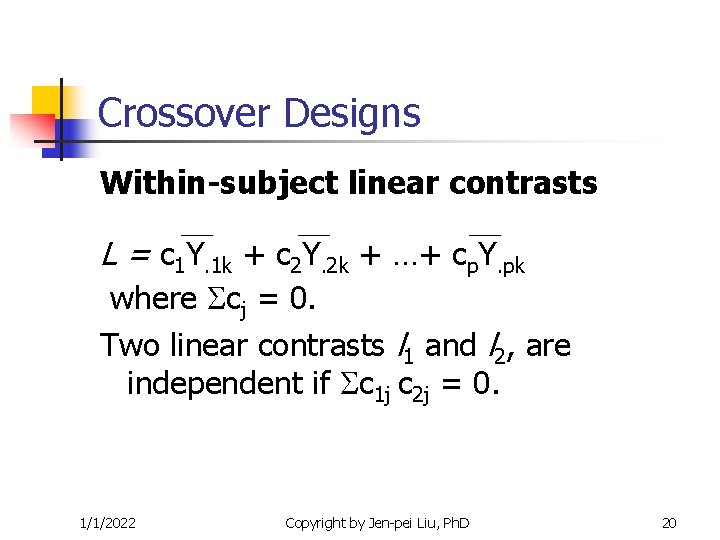
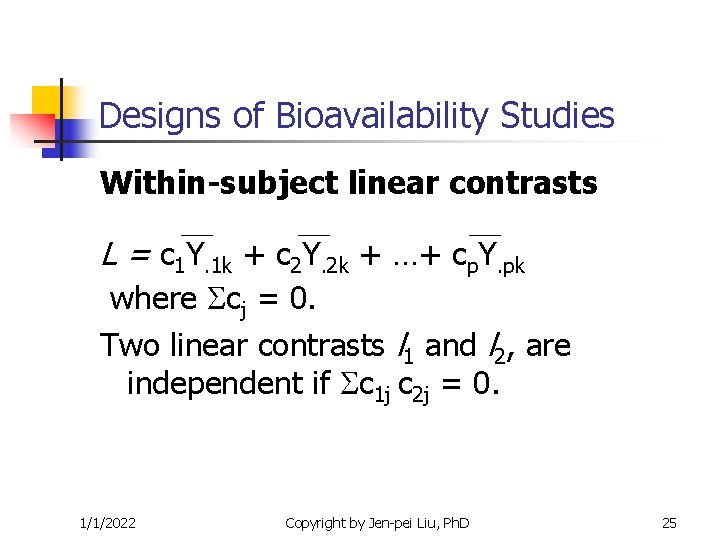
Crossover Designs Within-subject linear contrasts L = c 1 Y. 1 k + c 2 Y. 2 k + …+ cp. Y. pk where cj = 0. Two linear contrasts l 1 and l 2, are independent if c 1 j c 2 j = 0. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 20

Crossover Designs Complete crossover design: each sequence contains all formulations A g x p crossover design: There are g sequences of formulations administered at p different time periods. Washout period: Rest period between two treatment periods for which the effect of one formulation administered at one treatment period does not carry over to the next. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 21

Crossover Designs Direct drug effect: The effect that a drug product has during the period in which the drug is administered. Carryover effect: The effect that persists after the end of the dosing period. C-order carryover effect: The carryover effects last up to c treatment periods. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 22
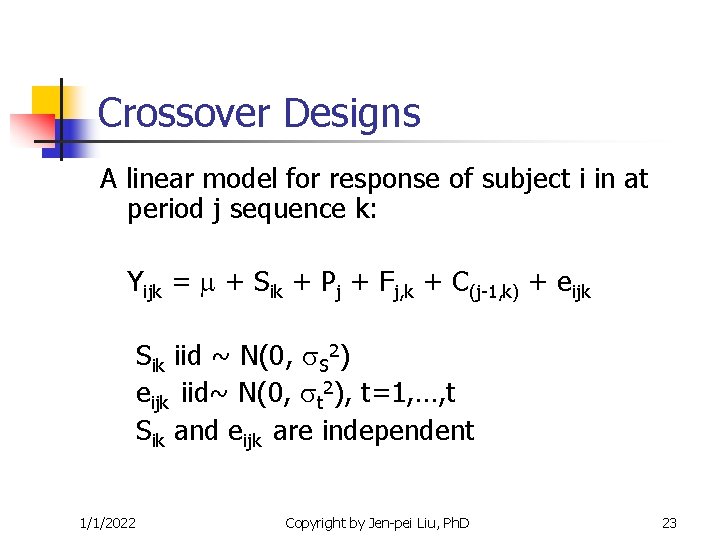
Crossover Designs A linear model for response of subject i in at period j sequence k: Yijk = + Sik + Pj + Fj, k + C(j-1, k) + eijk Sik iid ~ N(0, S 2) eijk iid~ N(0, t 2), t=1, …, t Sik and eijk are independent 1/1/2022 Copyright by Jen-pei Liu, Ph. D 23
Crossover Designs Observed means for period j in sequence k Y. jk = (1/nk) Yijk All fixed effects can be estimated based on gp sequence-by-period means (gp – 1) = (g-1) + (p-1) + (g-1)(p-1) overall = seq + period + seq*period 1/1/2022 Copyright by Jen-pei Liu, Ph. D 24
Designs of Bioavailability Studies Within-subject linear contrasts L = c 1 Y. 1 k + c 2 Y. 2 k + …+ cp. Y. pk where cj = 0. Two linear contrasts l 1 and l 2, are independent if c 1 j c 2 j = 0. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 25
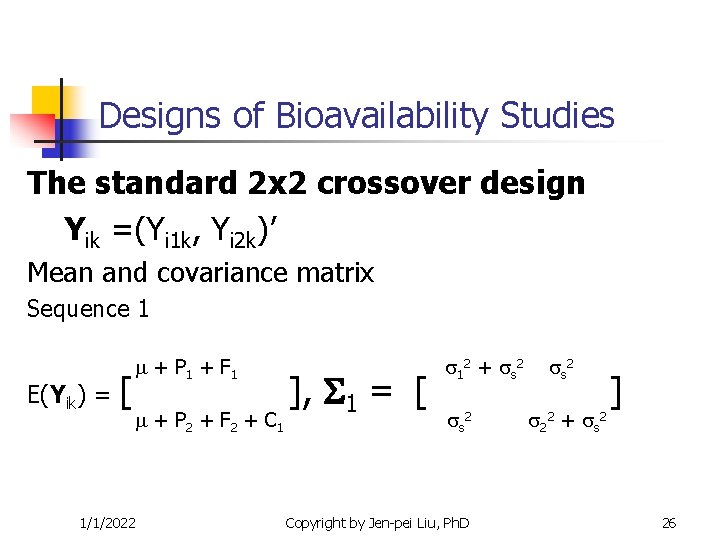
Designs of Bioavailability Studies The standard 2 x 2 crossover design Yik =(Yi 1 k, Yi 2 k)’ Mean and covariance matrix Sequence 1 E(Yik) = [ 1/1/2022 + P 1 + F 1 + P 2 + F 2 + C 1 ], 1 = [ 12 + s 2 Copyright by Jen-pei Liu, Ph. D s 2 22 + s 2 ] 26
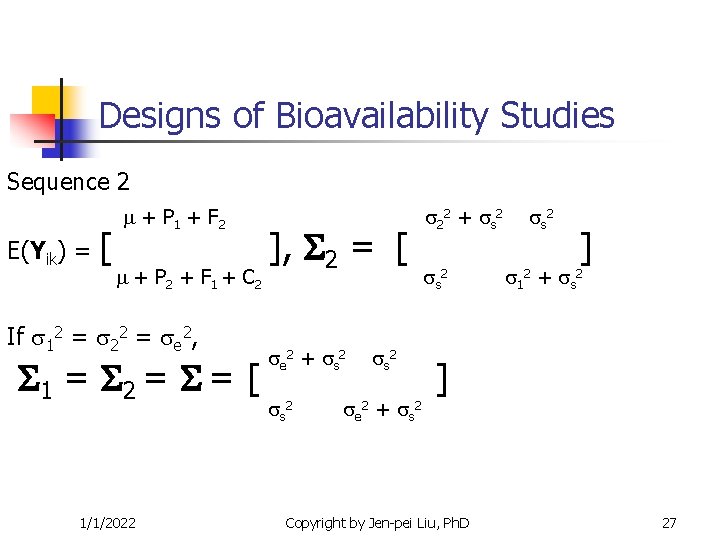
Designs of Bioavailability Studies Sequence 2 E(Yik) = [ + P 1 + F 2 + P 2 + F 1 + C 2 If 12 = 22 = e 2, 1 = 2 = = [ 1/1/2022 ], 2 = [ e 2 + s 2 e 2 + s 2 22 + s 2 ] 12 + s 2 ] Copyright by Jen-pei Liu, Ph. D 27
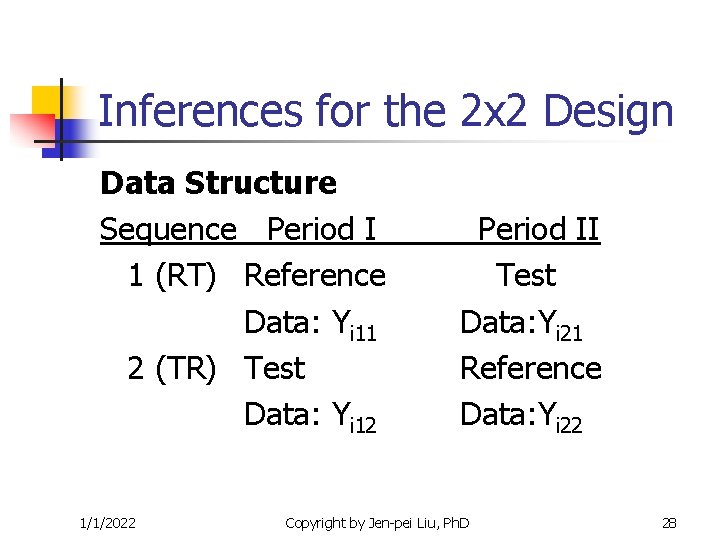
Inferences for the 2 x 2 Design Data Structure Sequence Period I 1 (RT) Reference Data: Yi 11 2 (TR) Test Data: Yi 12 1/1/2022 Period II Test Data: Yi 21 Reference Data: Yi 22 Copyright by Jen-pei Liu, Ph. D 28
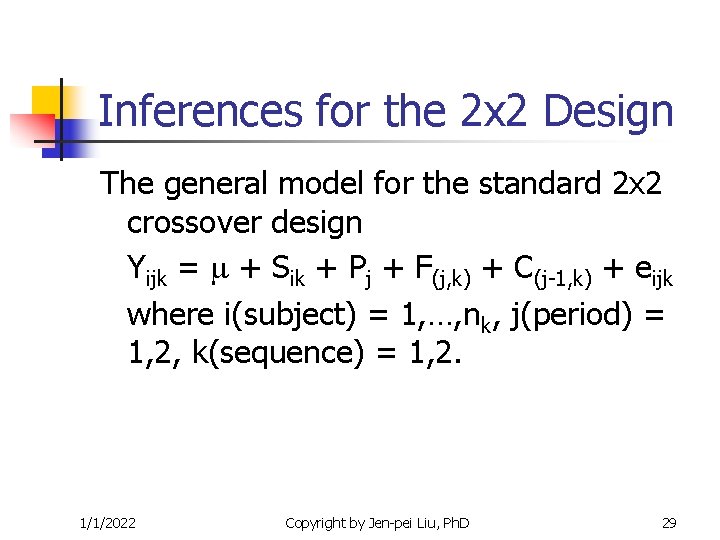
Inferences for the 2 x 2 Design The general model for the standard 2 x 2 crossover design Yijk = + Sik + Pj + F(j, k) + C(j-1, k) + eijk where i(subject) = 1, …, nk, j(period) = 1, 2, k(sequence) = 1, 2. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 29
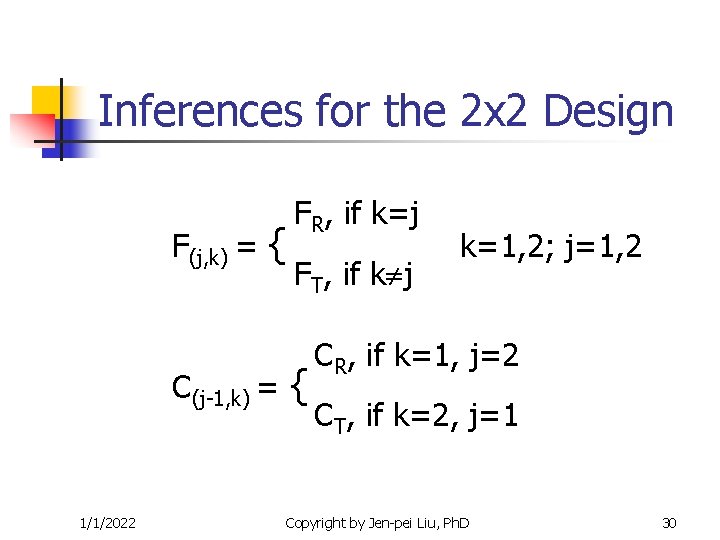
Inferences for the 2 x 2 Design F(j, k) = { C(j-1, k) = 1/1/2022 FR, if k=j FT, if k j { k=1, 2; j=1, 2 CR, if k=1, j=2 CT, if k=2, j=1 Copyright by Jen-pei Liu, Ph. D 30
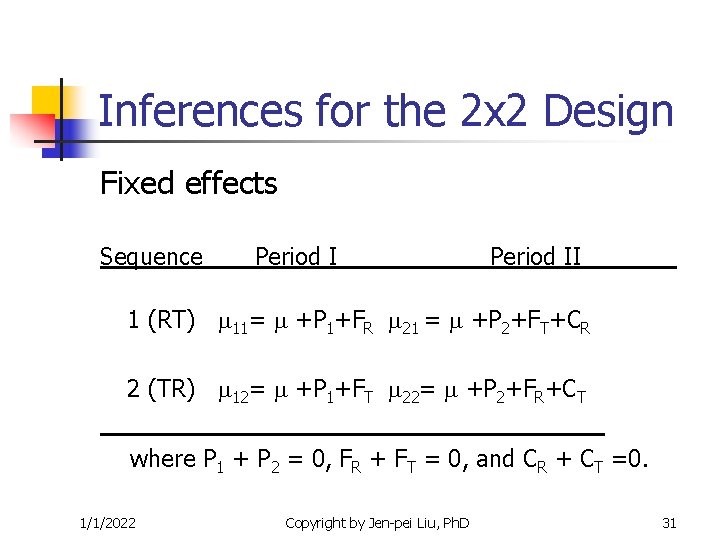
Inferences for the 2 x 2 Design Fixed effects Sequence Period II 1 (RT) 11= +P 1+FR 21 = +P 2+FT+CR 2 (TR) 12= +P 1+FT 22= +P 2+FR+CT where P 1 + P 2 = 0, FR + FT = 0, and CR + CT =0. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 31
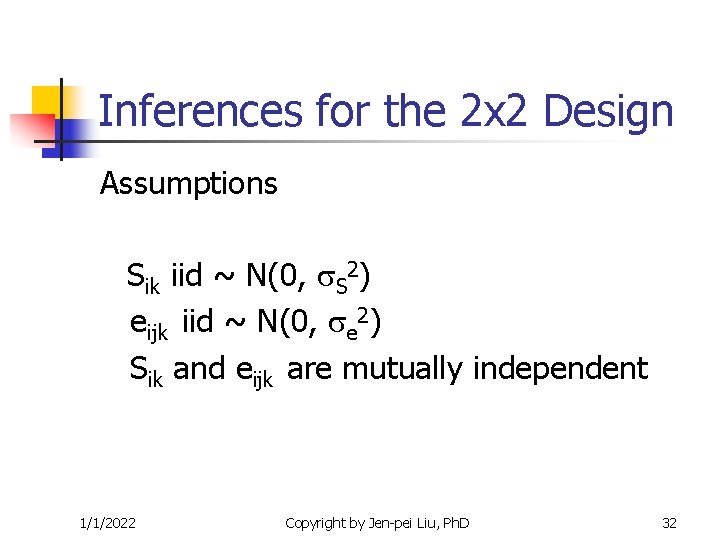
Inferences for the 2 x 2 Design Assumptions Sik iid ~ N(0, S 2) eijk iid ~ N(0, e 2) Sik and eijk are mutually independent 1/1/2022 Copyright by Jen-pei Liu, Ph. D 32
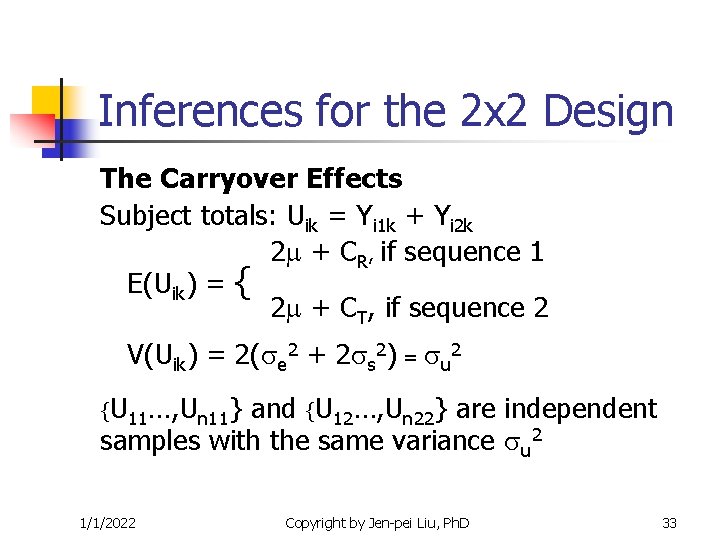
Inferences for the 2 x 2 Design The Carryover Effects Subject totals: Uik = Yi 1 k + Yi 2 k 2 + CR, if sequence 1 E(Uik) = { 2 + CT, if sequence 2 V(Uik) = 2( e 2 + 2 s 2) = u 2 {U 11…, Un 11} and {U 12…, Un 22} are independent samples with the same variance u 2 1/1/2022 Copyright by Jen-pei Liu, Ph. D 33
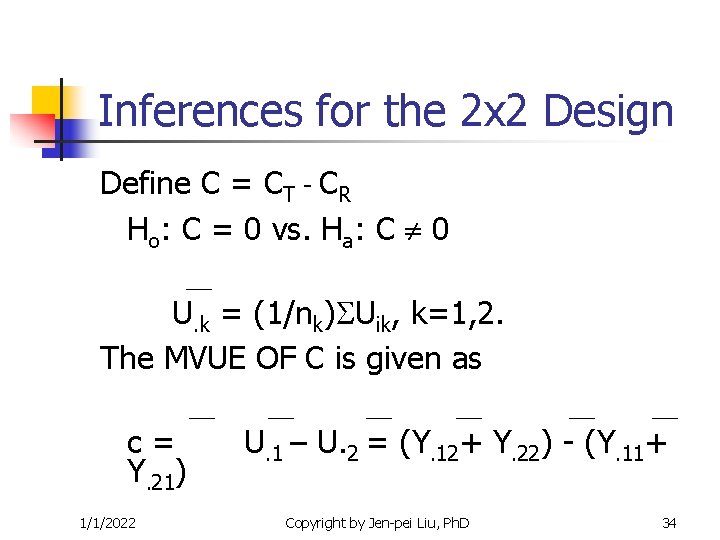
Inferences for the 2 x 2 Design Define C = CT - CR Ho: C = 0 vs. Ha: C 0 U. k = (1/nk) Uik, k=1, 2. The MVUE OF C is given as c= Y. 21) 1/1/2022 U. 1 – U. 2 = (Y. 12+ Y. 22) - (Y. 11+ Copyright by Jen-pei Liu, Ph. D 34
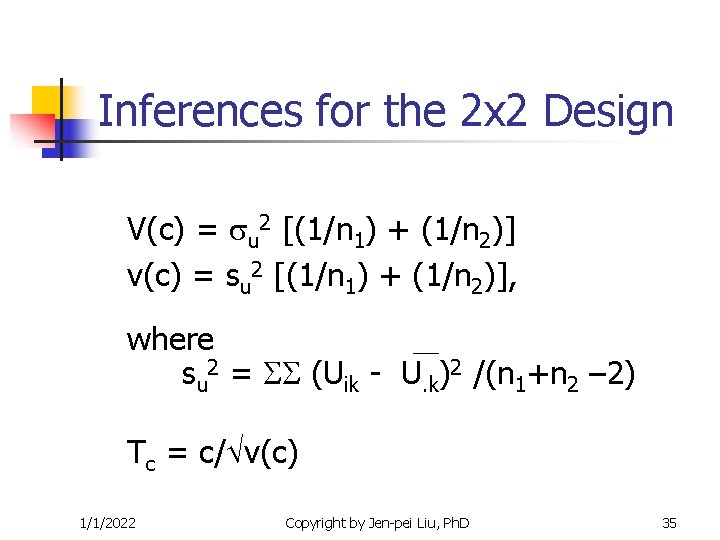
Inferences for the 2 x 2 Design V(c) = u 2 [(1/n 1) + (1/n 2)] v(c) = su 2 [(1/n 1) + (1/n 2)], where su 2 = (Uik - U. k)2 /(n 1+n 2 – 2) Tc = c/ v(c) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 35
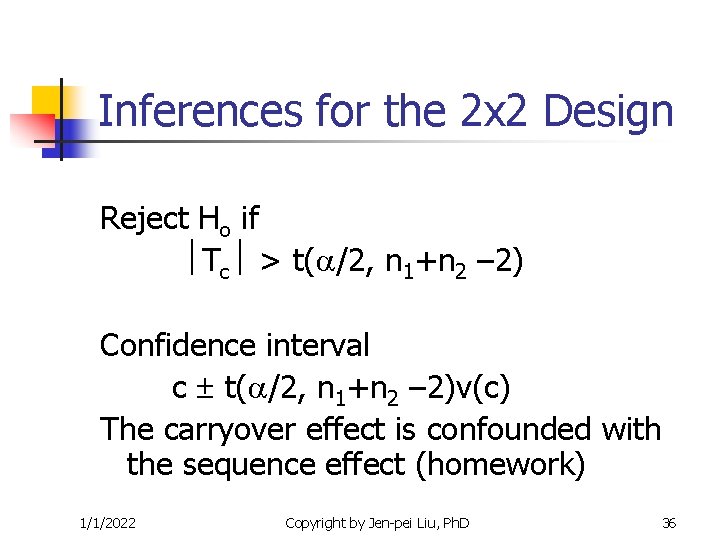
Inferences for the 2 x 2 Design Reject Ho if Tc > t( /2, n 1+n 2 – 2) Confidence interval c t( /2, n 1+n 2 – 2)v(c) The carryover effect is confounded with the sequence effect (homework) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 36
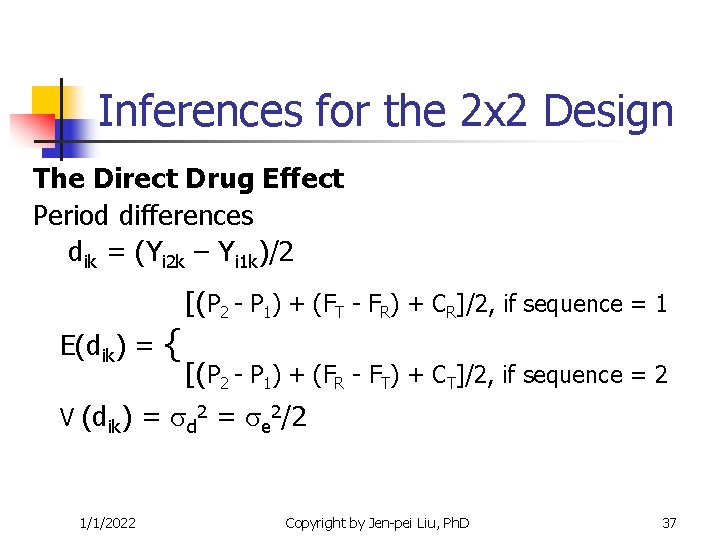
Inferences for the 2 x 2 Design The Direct Drug Effect Period differences dik = (Yi 2 k – Yi 1 k)/2 E(dik) = { [(P 2 - P 1) + (FT - FR) + CR]/2, if sequence = 1 [(P 2 - P 1) + (FR - FT) + CT]/2, if sequence = 2 V (dik) = d 2 = e 2/2 1/1/2022 Copyright by Jen-pei Liu, Ph. D 37
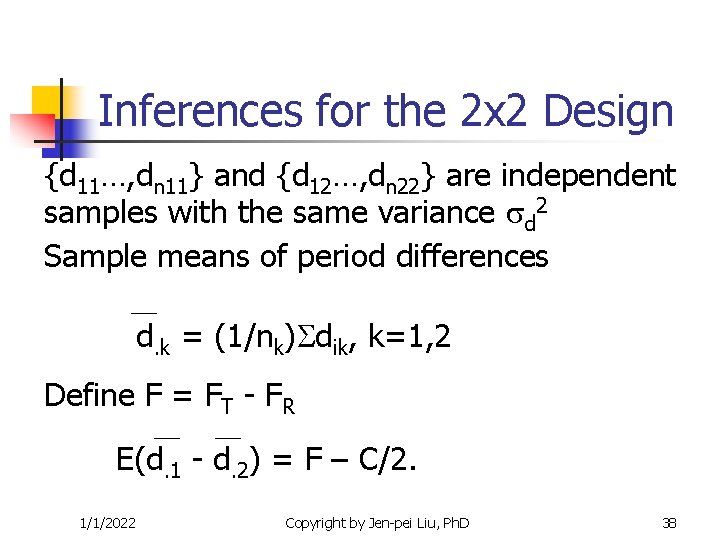
Inferences for the 2 x 2 Design {d 11…, dn 11} and {d 12…, dn 22} are independent samples with the same variance d 2 Sample means of period differences d. k = (1/nk) dik, k=1, 2 Define F = FT - FR E(d. 1 - d. 2) = F – C/2. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 38
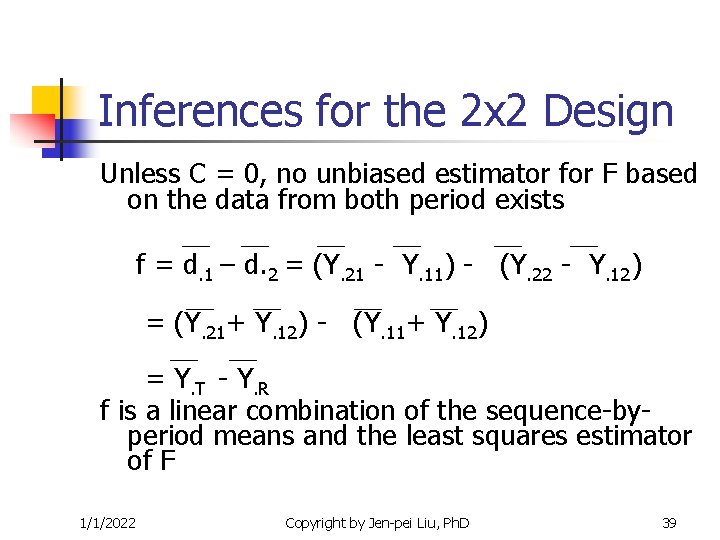
Inferences for the 2 x 2 Design Unless C = 0, no unbiased estimator for F based on the data from both period exists f = d. 1 – d. 2 = (Y. 21 - Y. 11) - (Y. 22 - Y. 12) = (Y. 21+ Y. 12) - (Y. 11+ Y. 12) = Y. T - Y. R f is a linear combination of the sequence-byperiod means and the least squares estimator of F 1/1/2022 Copyright by Jen-pei Liu, Ph. D 39
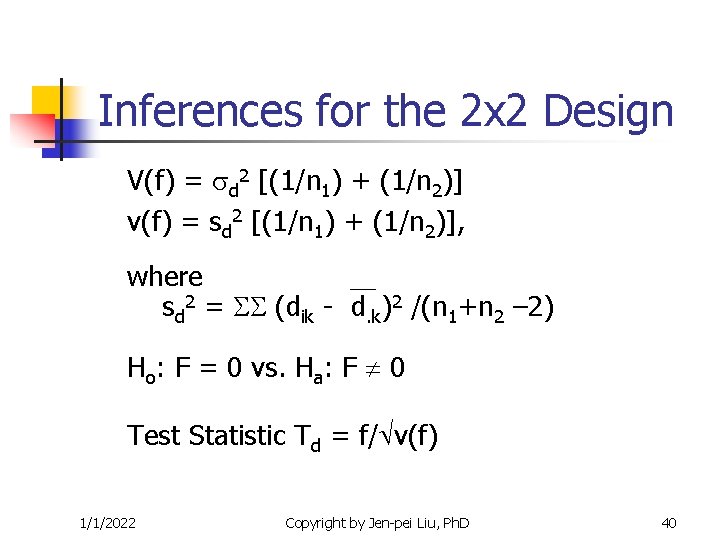
Inferences for the 2 x 2 Design V(f) = d 2 [(1/n 1) + (1/n 2)] v(f) = sd 2 [(1/n 1) + (1/n 2)], where 2 sd = (dik - d. k)2 /(n 1+n 2 – 2) Ho: F = 0 vs. Ha: F 0 Test Statistic Td = f/ v(f) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 40
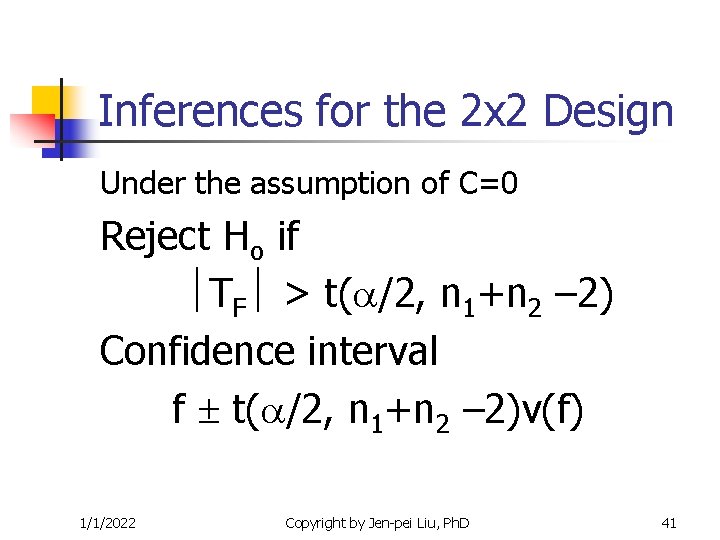
Inferences for the 2 x 2 Design Under the assumption of C=0 Reject Ho if TF > t( /2, n 1+n 2 – 2) Confidence interval f t( /2, n 1+n 2 – 2)v(f) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 41
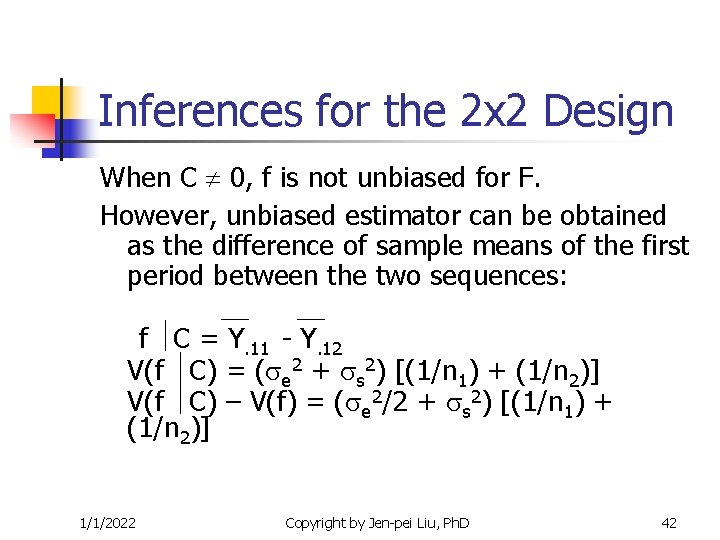
Inferences for the 2 x 2 Design When C 0, f is not unbiased for F. However, unbiased estimator can be obtained as the difference of sample means of the first period between the two sequences: f C = Y. 11 - Y. 12 V(f C) = ( e 2 + s 2) [(1/n 1) + (1/n 2)] V(f C) – V(f) = ( e 2/2 + s 2) [(1/n 1) + (1/n 2)] 1/1/2022 Copyright by Jen-pei Liu, Ph. D 42
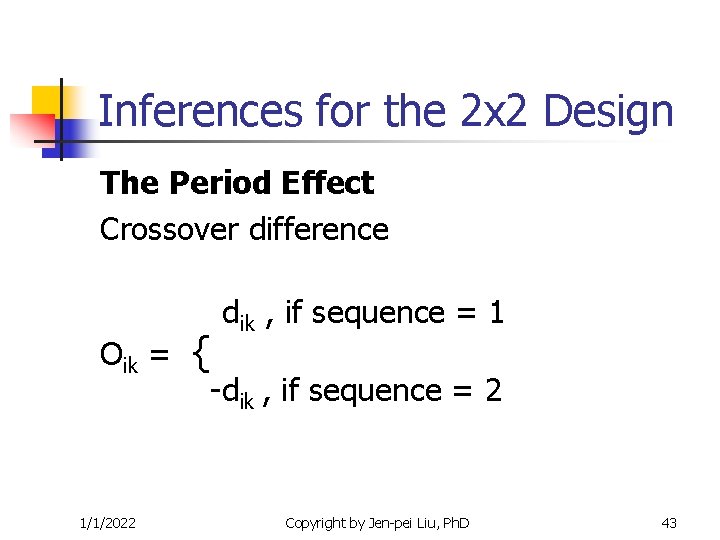
Inferences for the 2 x 2 Design The Period Effect Crossover difference Oik = 1/1/2022 { dik , if sequence = 1 -dik , if sequence = 2 Copyright by Jen-pei Liu, Ph. D 43
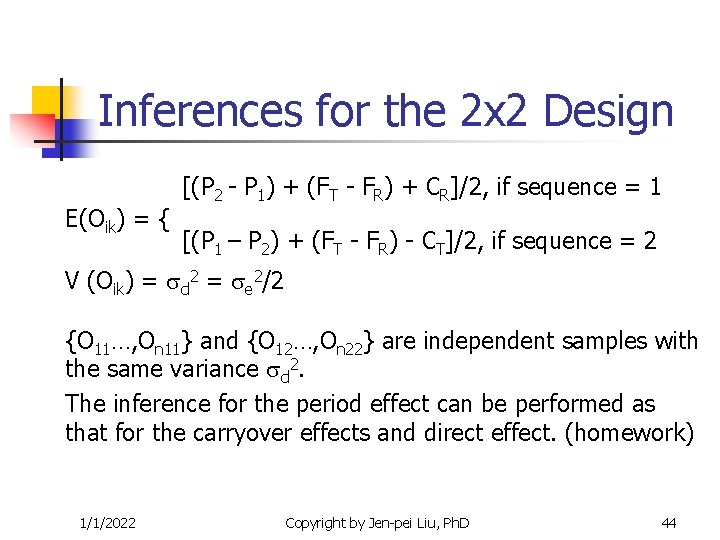
Inferences for the 2 x 2 Design E(Oik) = { [(P 2 - P 1) + (FT - FR) + CR]/2, if sequence = 1 [(P 1 – P 2) + (FT - FR) - CT]/2, if sequence = 2 V (Oik) = d 2 = e 2/2 {O 11…, On 11} and {O 12…, On 22} are independent samples with the same variance d 2. The inference for the period effect can be performed as that for the carryover effects and direct effect. (homework) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 44
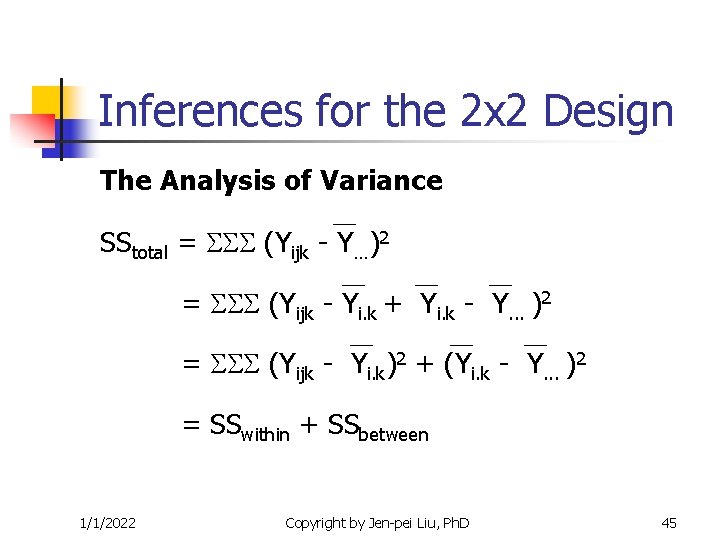
Inferences for the 2 x 2 Design The Analysis of Variance SStotal = (Yijk - Y…)2 = (Yijk - Yi. k + Yi. k - Y. . . )2 = (Yijk - Yi. k)2 + (Yi. k - Y. . . )2 = SSwithin + SSbetween 1/1/2022 Copyright by Jen-pei Liu, Ph. D 45
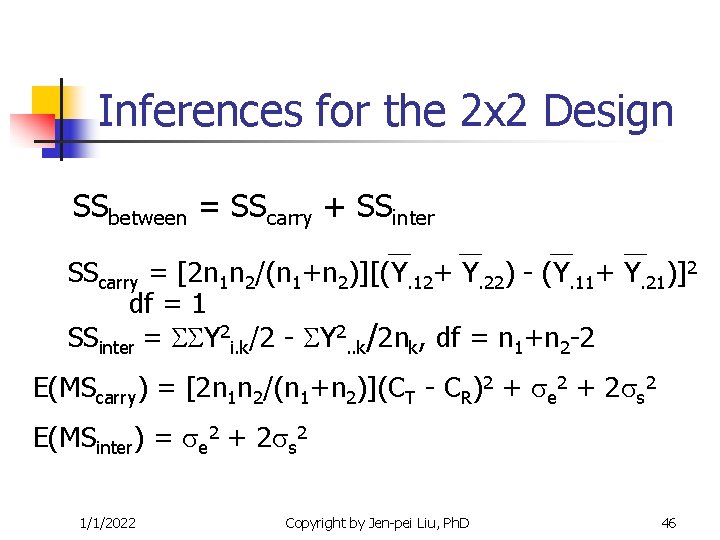
Inferences for the 2 x 2 Design SSbetween = SScarry + SSinter SScarry = [2 n 1 n 2/(n 1+n 2)][(Y. 12+ Y. 22) - (Y. 11+ Y. 21)]2 df = 1 SSinter = Y 2 i. k/2 - Y 2. . k/2 nk, df = n 1+n 2 -2 E(MScarry) = [2 n 1 n 2/(n 1+n 2)](CT - CR)2 + e 2 + 2 s 2 E(MSinter) = e 2 + 2 s 2 1/1/2022 Copyright by Jen-pei Liu, Ph. D 46
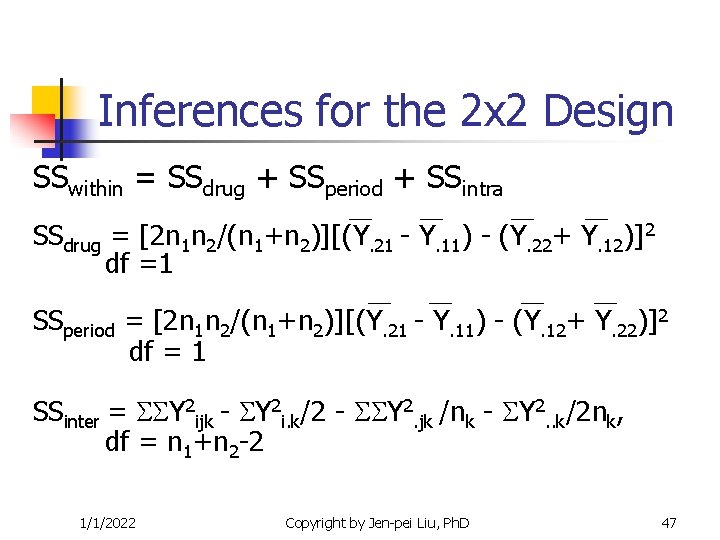
Inferences for the 2 x 2 Design SSwithin = SSdrug + SSperiod + SSintra SSdrug = [2 n 1 n 2/(n 1+n 2)][(Y. 21 - Y. 11) - (Y. 22+ Y. 12)]2 df =1 SSperiod = [2 n 1 n 2/(n 1+n 2)][(Y. 21 - Y. 11) - (Y. 12+ Y. 22)]2 df = 1 SSinter = Y 2 ijk - Y 2 i. k/2 - Y 2. jk /nk - Y 2. . k/2 nk, df = n 1+n 2 -2 1/1/2022 Copyright by Jen-pei Liu, Ph. D 47
![Inferences for the 2 x 2 Design E(MSdrug)=[2 n 1 n 2/(n 1+n 2)][(FT Inferences for the 2 x 2 Design E(MSdrug)=[2 n 1 n 2/(n 1+n 2)][(FT](http://slidetodoc.com/presentation_image_h2/4687b04f8700875a25376f6e5348e845/image-48.jpg)
Inferences for the 2 x 2 Design E(MSdrug)=[2 n 1 n 2/(n 1+n 2)][(FT - FR)+(CT - CR)]2+ e 2 E(MSperiod) = [2 n 1 n 2/(n 1+n 2)](P 2 – P 1)2 + e 2 E(MSintra) = e 2 1/1/2022 Copyright by Jen-pei Liu, Ph. D 48
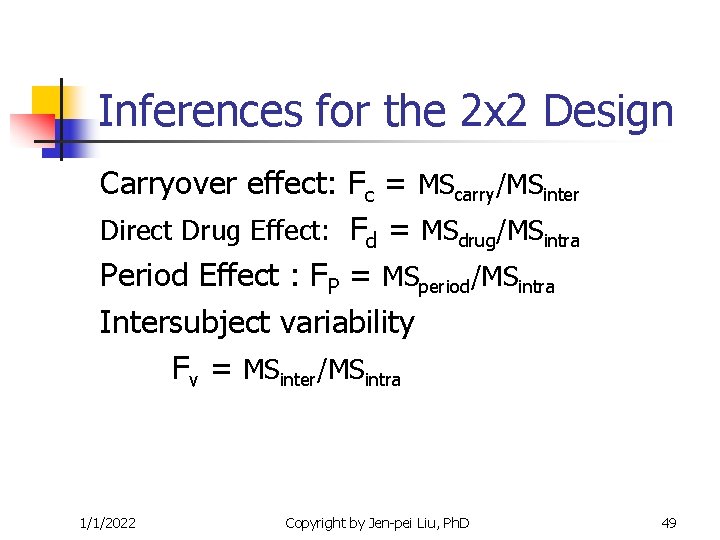
Inferences for the 2 x 2 Design Carryover effect: Fc = MScarry/MSinter Direct Drug Effect: Fd = MSdrug/MSintra Period Effect : FP = MSperiod/MSintra Intersubject variability Fv = MSinter/MSintra 1/1/2022 Copyright by Jen-pei Liu, Ph. D 49

Factorial Designs Two or more treatments are evaluated simultaneously in the same sets of patients via various of combinations of two treatments. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 50
Factorial Designs n n Factors: Drugs Levels: Doses Treatments: Combinations of drugs and doses Design: parallel, crossover, or a combination of parallel and crossover 1/1/2022 Copyright by Jen-pei Liu, Ph. D 51
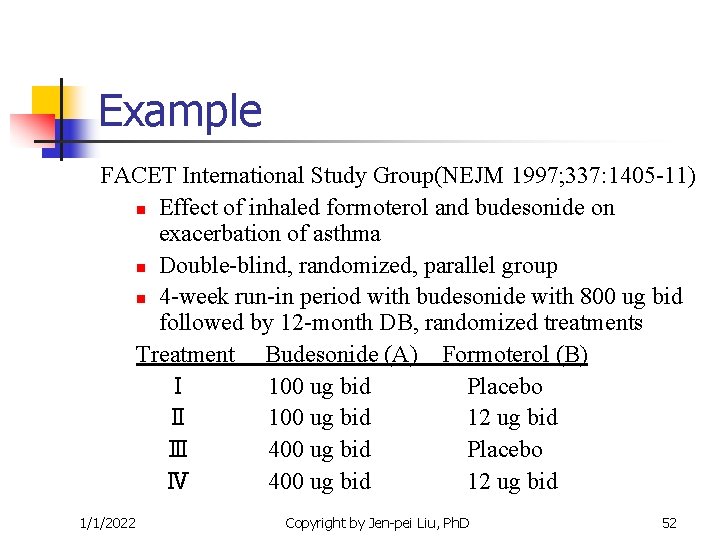
Example FACET International Study Group(NEJM 1997; 337: 1405 -11) n Effect of inhaled formoterol and budesonide on exacerbation of asthma n Double-blind, randomized, parallel group n 4 -week run-in period with budesonide with 800 ug bid followed by 12 -month DB, randomized treatments Treatment Budesonide (A) Formoterol (B) Ⅰ 100 ug bid Placebo Ⅱ 100 ug bid 12 ug bid Ⅲ 400 ug bid Placebo Ⅳ 400 ug bid 12 ug bid 1/1/2022 Copyright by Jen-pei Liu, Ph. D 52
1/1/2022 Copyright by Jen-pei Liu, Ph. D 53
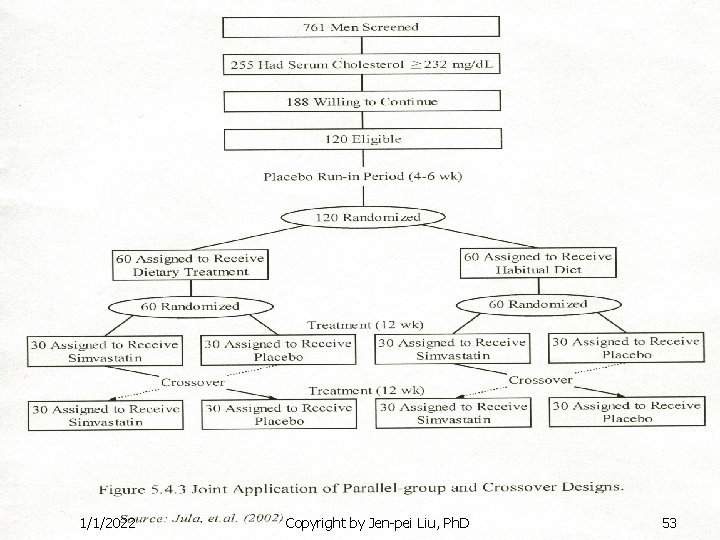
Example n n Parallel group design: a comparison between dietary treatment (Mediterranean-type diet) and habitual diet Crossover design: a comparison between simvastatin (a cholesterol lowering agent) and placebo 1/1/2022 Copyright by Jen-pei Liu, Ph. D 54
Example n Advantages n n can efficiently use patients for evaluation of efficacy of both treatments can investigate the joint treatment effects can establish dose-response relationship of a combination drug product Drawbacks n n 1/1/2022 Difficult to implement because of large number of treatment groups Lack of power for interaction Copyright by Jen-pei Liu, Ph. D 55
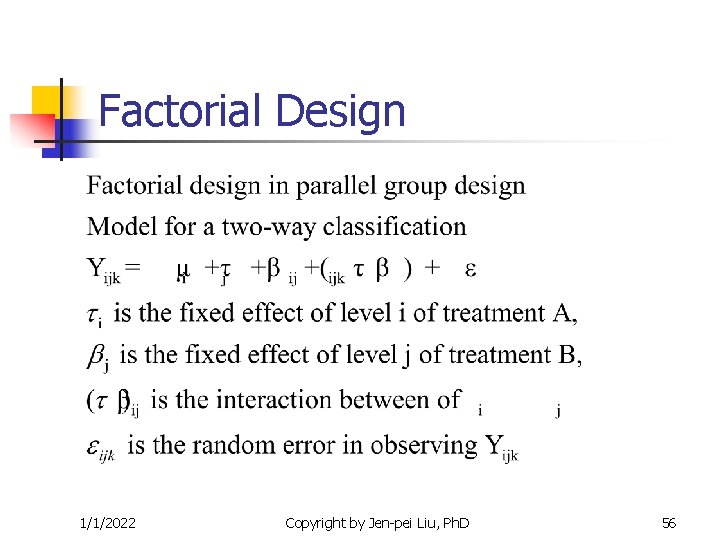
Factorial Design 1/1/2022 Copyright by Jen-pei Liu, Ph. D 56
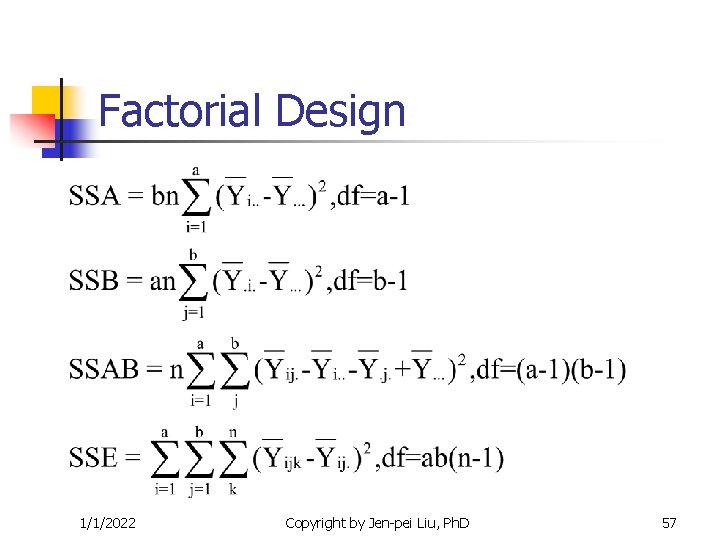
Factorial Design 1/1/2022 Copyright by Jen-pei Liu, Ph. D 57
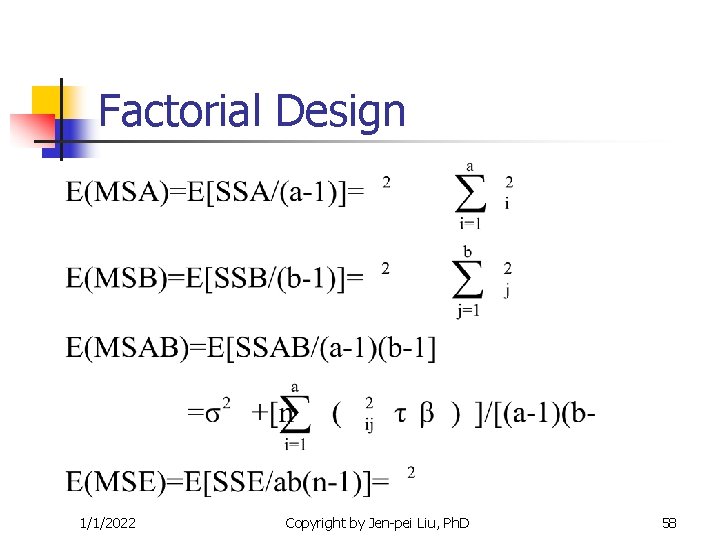
Factorial Design 1/1/2022 Copyright by Jen-pei Liu, Ph. D 58
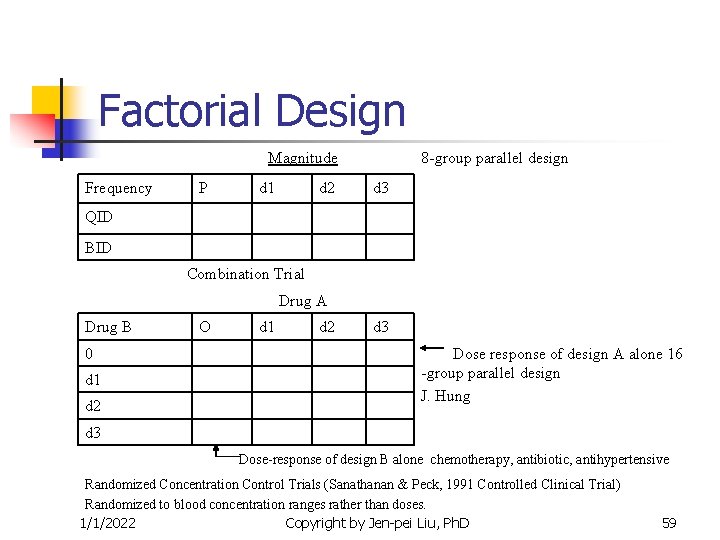
Factorial Design Magnitude Frequency P d 1 d 2 8 -group parallel design d 3 QID BID Combination Trial Drug A Drug B 0 d 1 d 2 O d 1 d 2 d 3 Dose response of design A alone 16 -group parallel design J. Hung d 3 Dose-response of design B alone chemotherapy, antibiotic, antihypertensive Randomized Concentration Control Trials (Sanathanan & Peck, 1991 Controlled Clinical Trial) Randomized to blood concentration ranges rather than doses. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 59
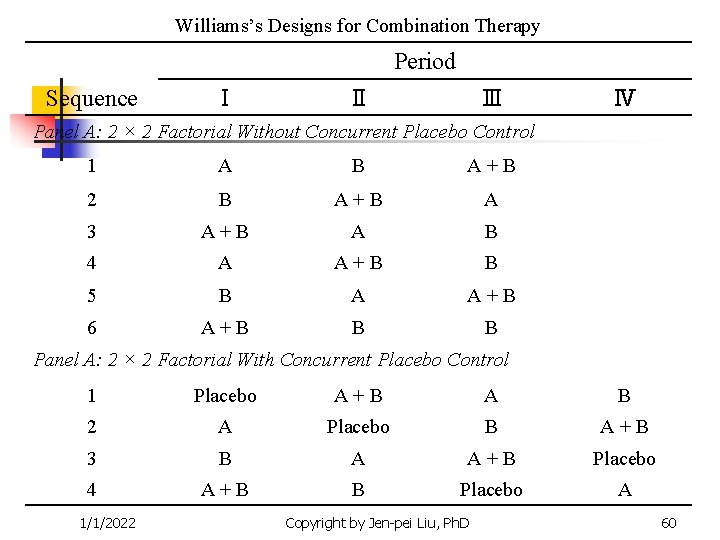
Williams’s Designs for Combination Therapy Period Sequence Ⅰ Ⅱ Ⅲ Ⅳ Panel A: 2 × 2 Factorial Without Concurrent Placebo Control 1 A B A+B 2 B A+B A 3 A+B A B 4 A A+B B 5 B A A+B 6 A+B B B Panel A: 2 × 2 Factorial With Concurrent Placebo Control 1 Placebo A+B A B 2 A Placebo B A+B 3 B A A+B Placebo 4 A+B B Placebo A 1/1/2022 Copyright by Jen-pei Liu, Ph. D 60
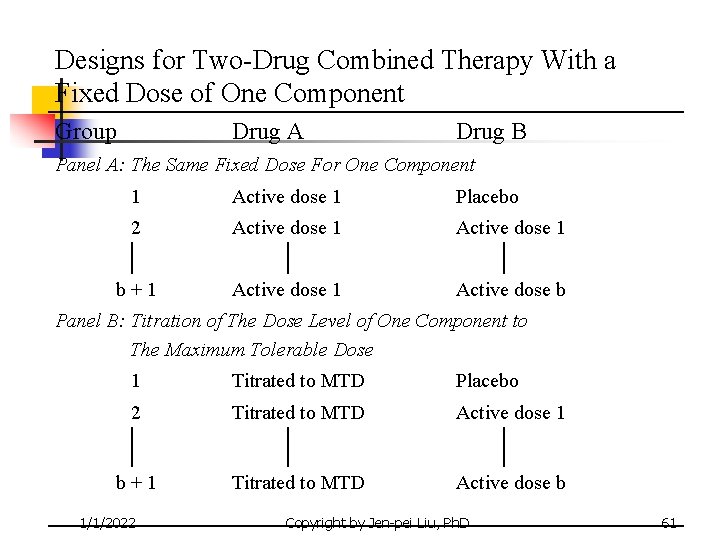
Designs for Two-Drug Combined Therapy With a Fixed Dose of One Component Group Drug A Drug B Panel A: The Same Fixed Dose For One Component 1 Active dose 1 Placebo 2 Active dose 1 b+1 Active dose b Panel B: Titration of The Dose Level of One Component to The Maximum Tolerable Dose 1 Titrated to MTD Placebo 2 Titrated to MTD Active dose 1 b+1 Titrated to MTD Active dose b 1/1/2022 Copyright by Jen-pei Liu, Ph. D 61
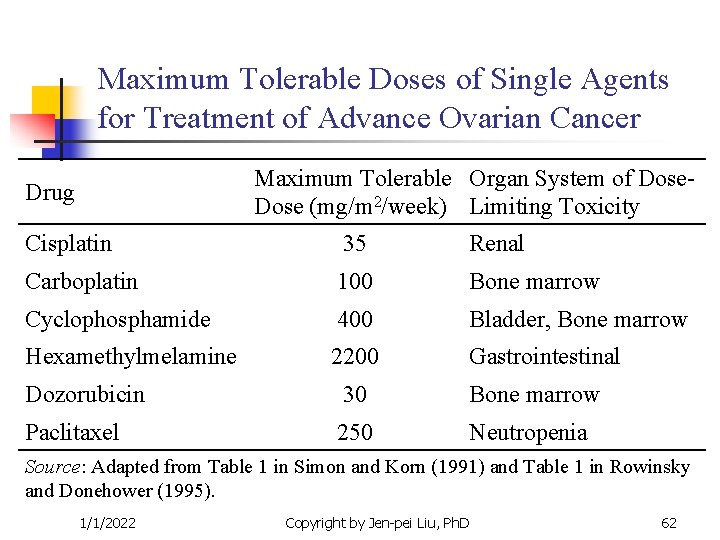
Maximum Tolerable Doses of Single Agents for Treatment of Advance Ovarian Cancer Maximum Tolerable Organ System of Dose (mg/m 2/week) Limiting Toxicity Drug Cisplatin 35 Renal Carboplatin 100 Bone marrow Cyclophosphamide 400 Bladder, Bone marrow Hexamethylmelamine 2200 Gastrointestinal Dozorubicin 30 Bone marrow Paclitaxel 250 Neutropenia Source: Adapted from Table 1 in Simon and Korn (1991) and Table 1 in Rowinsky and Donehower (1995). 1/1/2022 Copyright by Jen-pei Liu, Ph. D 62
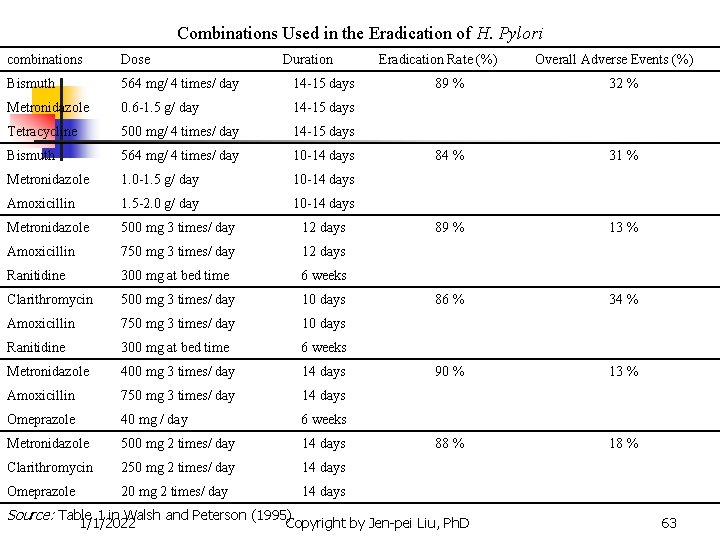
Combinations Used in the Eradication of H. Pylori combinations Dose Duration Bismuth 564 mg/ 4 times/ day 14 -15 days Metronidazole 0. 6 -1. 5 g/ day 14 -15 days Tetracycline 500 mg/ 4 times/ day 14 -15 days Bismuth 564 mg/ 4 times/ day 10 -14 days Metronidazole 1. 0 -1. 5 g/ day 10 -14 days Amoxicillin 1. 5 -2. 0 g/ day 10 -14 days Metronidazole 500 mg 3 times/ day 12 days Amoxicillin 750 mg 3 times/ day 12 days Ranitidine 300 mg at bed time 6 weeks Clarithromycin 500 mg 3 times/ day 10 days Amoxicillin 750 mg 3 times/ day 10 days Ranitidine 300 mg at bed time 6 weeks Metronidazole 400 mg 3 times/ day 14 days Amoxicillin 750 mg 3 times/ day 14 days Omeprazole 40 mg / day 6 weeks Metronidazole 500 mg 2 times/ day 14 days Clarithromycin 250 mg 2 times/ day 14 days Omeprazole 20 mg 2 times/ day 14 days Eradication Rate (%) Overall Adverse Events (%) 89 % 32 % 84 % 31 % 89 % 13 % 86 % 34 % 90 % 13 % 88 % 18 % Source: Table 1 in Walsh and Peterson (1995). 1/1/2022 Copyright by Jen-pei Liu, Ph. D 63
Dose Titration Designs n n n A set of pre-determined dose levels A set of pre-specified criteria of responders Initiation with a placebo washout period Titration up according to the pre-specified Advantages n n n Simulate real clinical practices Require fewer patients provide information where toxicity at higher dose levels is relatively unclear 1/1/2022 Copyright by Jen-pei Liu, Ph. D 64
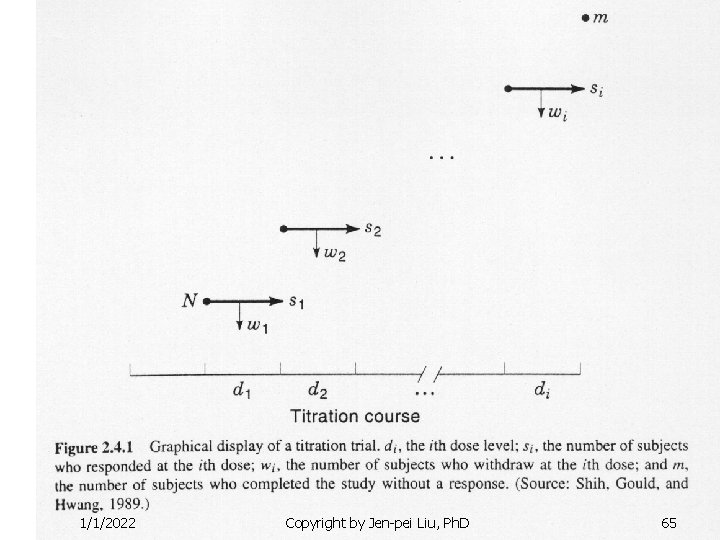
1/1/2022 Copyright by Jen-pei Liu, Ph. D 65
Dose Titration Designs n Drawbacks n n n n Unable to blind the dose levels Treatment effects are confounded with time Unable to separate and estimate carryover effects Should include a concurrent parallel placebo group Overestimation of the necessary dose Formal statistical procedures are yet to be developed except for binary data Results should be considered exploratory rather confirmatory 1/1/2022 Copyright by Jen-pei Liu, Ph. D 66
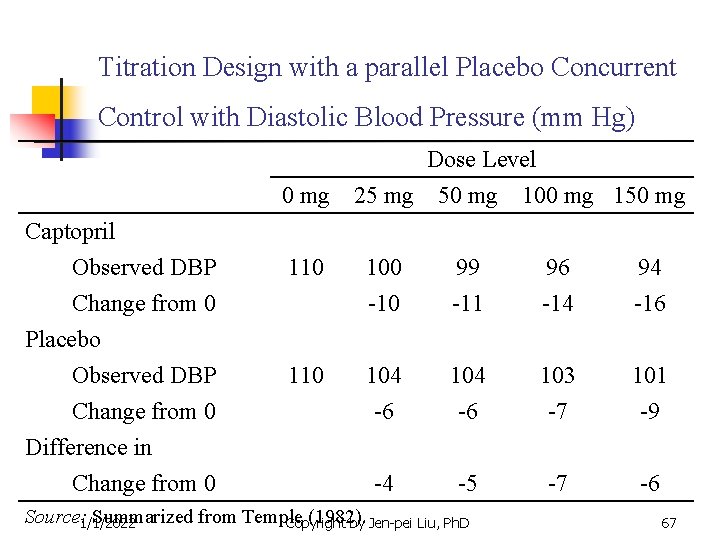
Titration Design with a parallel Placebo Concurrent Control with Diastolic Blood Pressure (mm Hg) Dose Level 0 mg 25 mg 50 mg 100 mg 150 mg Captopril Observed DBP Change from 0 Placebo Observed DBP Change from 0 Difference in Change from 0 110 100 99 96 94 -10 -11 -14 -16 104 -6 103 -7 101 -9 -4 -5 -7 -6 Source: 1/1/2022 Summarized from Temple (1982). Copyright by Jen-pei Liu, Ph. D 67

Forced Dose-Escalation Designs n Drugs have undesirable but reversible safety concerns n Drugs are not efficacious at lower dose levels n n n Criteria for escalation of dose levels are some safety limiting factors A significant number of dropouts is expected All patients with no pre-defined safety problems are forced to receive the next higher dose in the subsequent dosing period 1/1/2022 Copyright by Jen-pei Liu, Ph. D 68
Example Farlow, et al (JAMA 1992; 268: 2523 -2529) Knapp, et al (JAMA 1992; 271: 985 -991) n Tacrine for th treatment of patients with mild to moderate dementia of probable Alzheimer’s disease n Induction of elevation of alanine aminotransferase (ALT) in 43%54% of patient over the upper limit of the normal range and in 28% of patients over three times the upper limits n The two-adequate well-controlled studies generated the substantial evidence for approval of tacrine employed the forced doseescalation design n Randomized, DB, placebo-controlled n Parallel groups as well as forced dose-escalation within each group 1/1/2022 Copyright by Jen-pei Liu, Ph. D 69
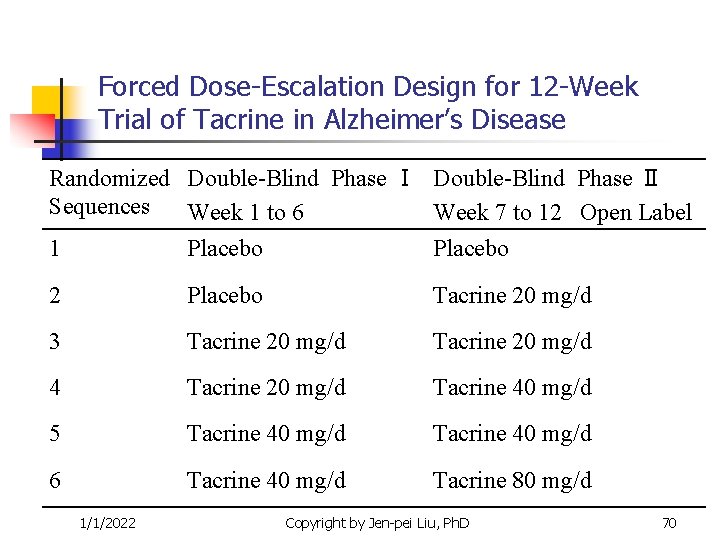
Forced Dose-Escalation Design for 12 -Week Trial of Tacrine in Alzheimer’s Disease Randomized Double-Blind Phase Ⅰ Double-Blind Phase Ⅱ Sequences Week 1 to 6 Week 7 to 12 Open Label 1 Placebo 2 Placebo Tacrine 20 mg/d 3 Tacrine 20 mg/d 4 Tacrine 20 mg/d Tacrine 40 mg/d 5 Tacrine 40 mg/d 6 Tacrine 40 mg/d Tacrine 80 mg/d 1/1/2022 Copyright by Jen-pei Liu, Ph. D 70
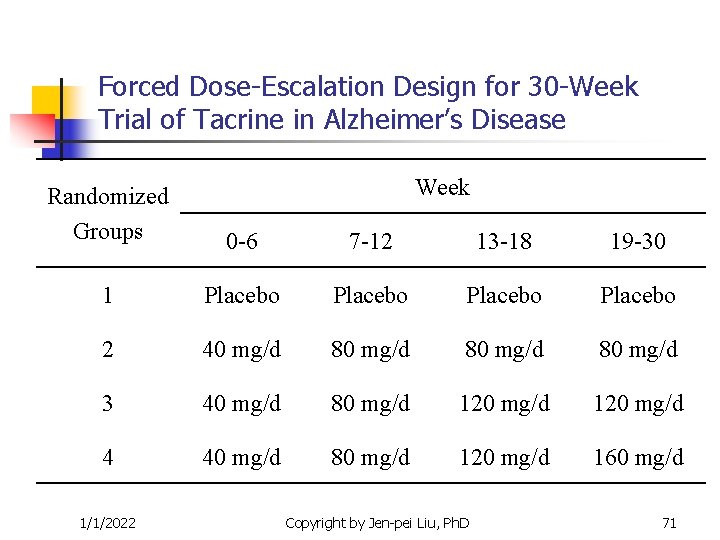
Forced Dose-Escalation Design for 30 -Week Trial of Tacrine in Alzheimer’s Disease Week Randomized Groups 0 -6 7 -12 13 -18 19 -30 1 Placebo 2 40 mg/d 80 mg/d 3 40 mg/d 80 mg/d 120 mg/d 4 40 mg/d 80 mg/d 120 mg/d 160 mg/d 1/1/2022 Copyright by Jen-pei Liu, Ph. D 71
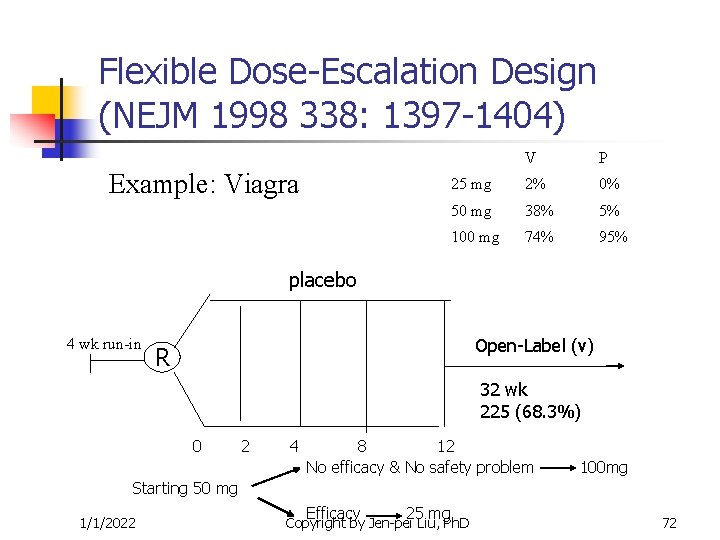
Flexible Dose-Escalation Design (NEJM 1998 338: 1397 -1404) Example: Viagra V P 25 mg 2% 0% 50 mg 38% 5% 100 mg 74% 95% placebo 4 wk run-in Open-Label (v) R 32 wk 225 (68. 3%) 0 2 4 8 12 No efficacy & No safety problem 100 mg Starting 50 mg 1/1/2022 Efficacy 25 mg Copyright by Jen-pei Liu, Ph. D 72

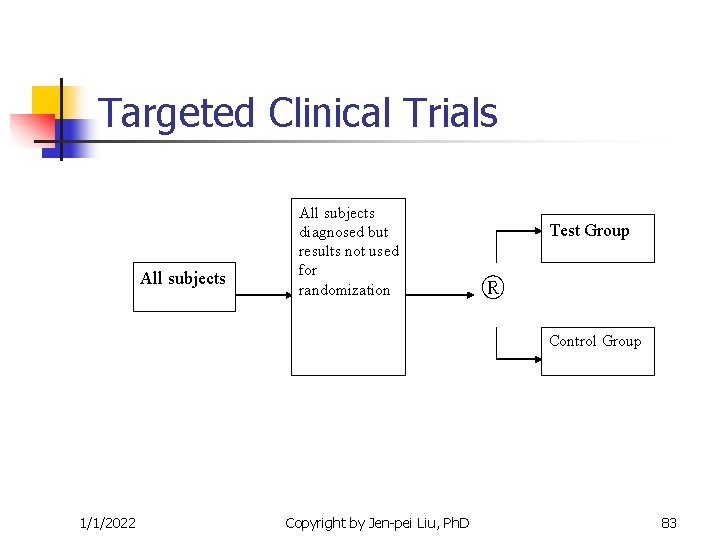
Targeted Clinical Trials n n n Therapeutic agents are molecularly targeted to protein products of genes that are disregulated in the patients with the disease. Identify the candidates in whom the agents is likely to be beneficial in the early phase of the study This early phase is called the Enrichment phase 1/1/2022 Copyright by Jen-pei Liu, Ph. D 73

Targeted Clinical Trials n n The identified candidates are then evaluated by the standard design for adequate wellcontrolled studies Enrichment Designs consist of two phases n n n Enrichment phase Randomized, double-blind, placebo-controlled phase Predictability of short-term response for longterm efficacy 1/1/2022 Copyright by Jen-pei Liu, Ph. D 74

Targeted Clinical Trials Imatinib mesylate (Gleevec) for Chronic Myelogenous Leukemia (CML) and gastrointesitnal stromal tumors (GIST) n Philadelphia (Ph+) chromosome from reciprocal translocation of long arms of 9 and 22 in 90% of patients with CML n Formation of BCR-ABL fusion gene BCR-ABL tyrosine kinase CML n KIT proto-oncogene transmembrane receptor KIT GIGS n Kantarjian et al. 2002; Demetri, et al. , 2002 1/1/2022 Copyright by Jen-pei Liu, Ph. D 75 n

Targeted Clinical Trials n n Other examples HER 2 gene in metastatic breast cancer - Herceptin requirement of screening the patients with overexpressed HER 2 level (Slamon, 2001). Estrogen receptor ploymorphism - Estrogen Replacement Atherosclerosis trial (ERA, Herrington, et al, 2002): a total of 9 SNPs were identified and interaction between treatment of HRT and some of SNPs in elevation of lipid levels is suggested Sample size determination: Fijal, et al. (2000) and Maitournam and Simon (2005). 1/1/2022 Copyright by Jen-pei Liu, Ph. D 76

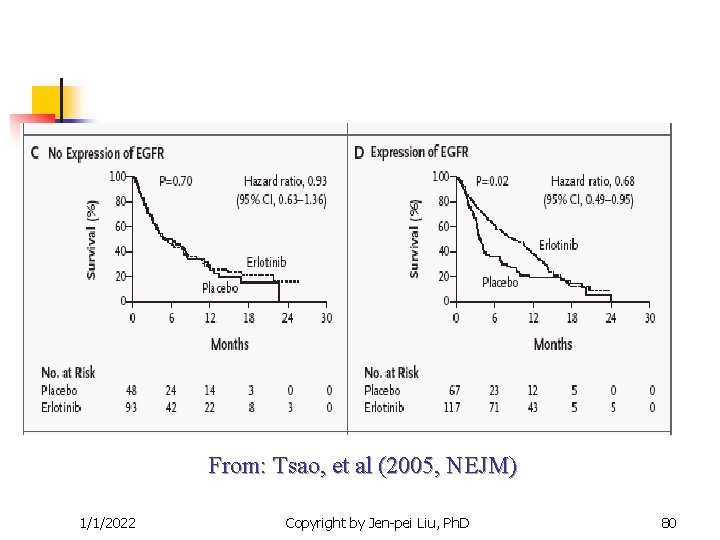
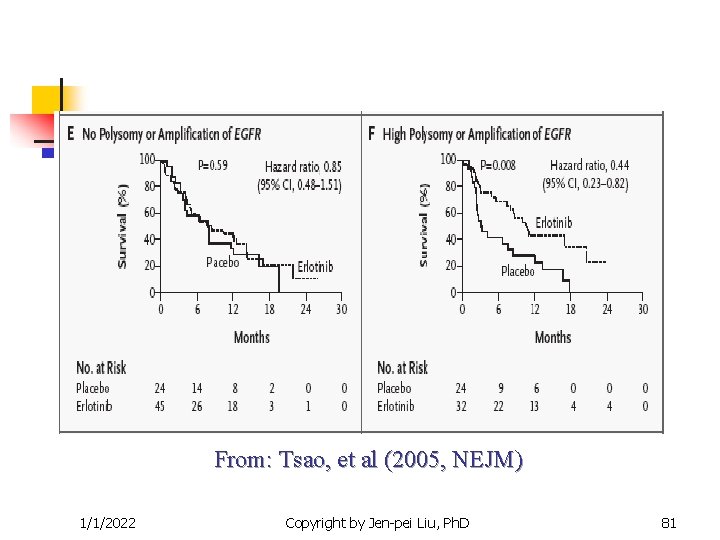
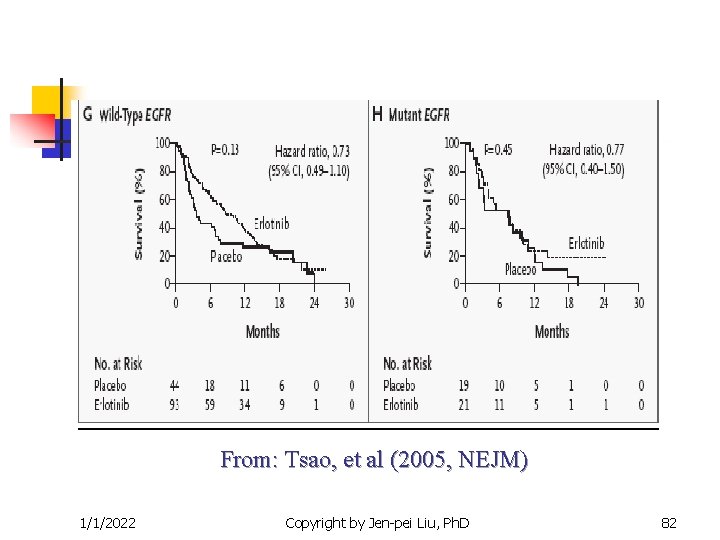
Targeted Clinical Trials n n Other examples The epidermal growth factor receptor (EGFR) inhibitor for the non-small cell lung cancer. Iressa (gefitnib) and Tarceva (Erlotinib) are targted at the EGFR pathway. Efficacy is correlated to race number of gene copies protein expression EGFR mutation Gappuzzo et al. (JNCI, 2005), Tsao, et al (NEJM, 2005) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 77
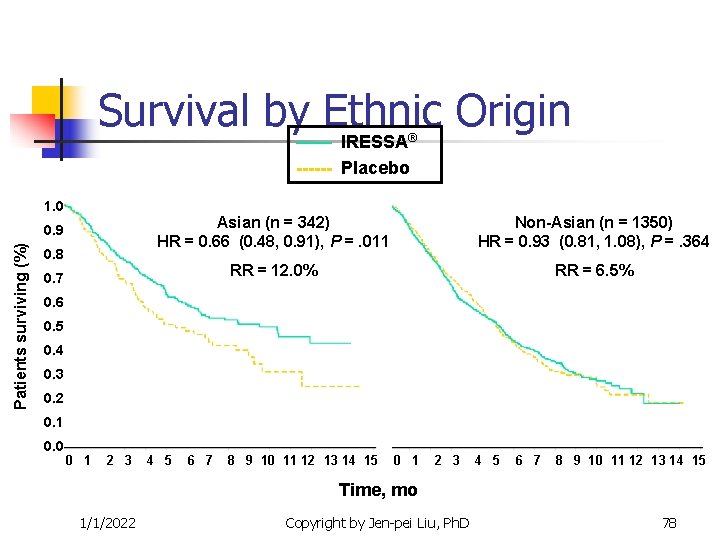
Survival by——Ethnic Origin IRESSA ® ------ Placebo 1. 0 Patients surviving (%) 0. 9 0. 8 Asian (n = 342) HR = 0. 66 (0. 48, 0. 91), P =. 011 Non-Asian (n = 1350) HR = 0. 93 (0. 81, 1. 08), P =. 364 RR = 12. 0% RR = 6. 5% 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Time, mo 1/1/2022 Copyright by Jen-pei Liu, Ph. D 78
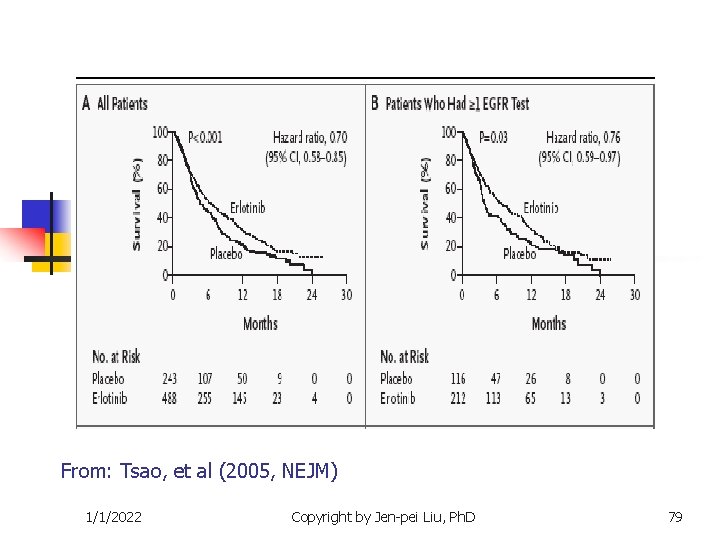
From: Tsao, et al (2005, NEJM) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 79
From: Tsao, et al (2005, NEJM) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 80
From: Tsao, et al (2005, NEJM) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 81
From: Tsao, et al (2005, NEJM) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 82
Targeted Clinical Trials All subjects diagnosed but results not used for randomization Test Group ® Control Group 1/1/2022 Copyright by Jen-pei Liu, Ph. D 83
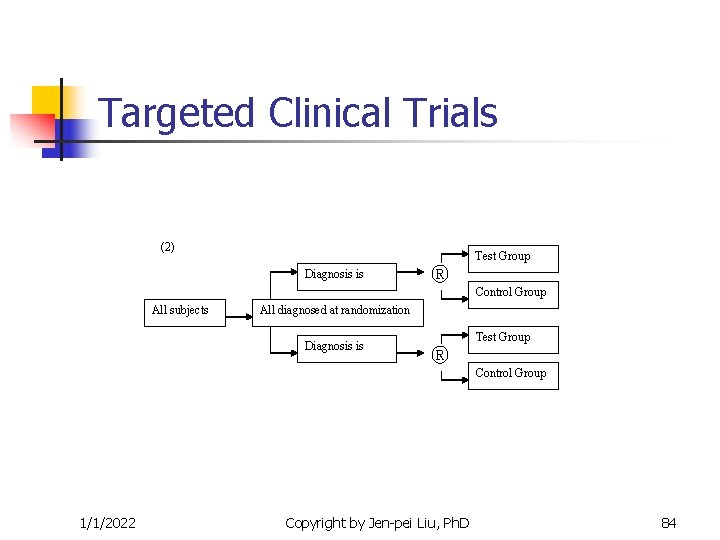
Targeted Clinical Trials (2) Diagnosis is ® Test Group Control Group All subjects All diagnosed at randomization Diagnosis is ® Test Group Control Group 1/1/2022 Copyright by Jen-pei Liu, Ph. D 84
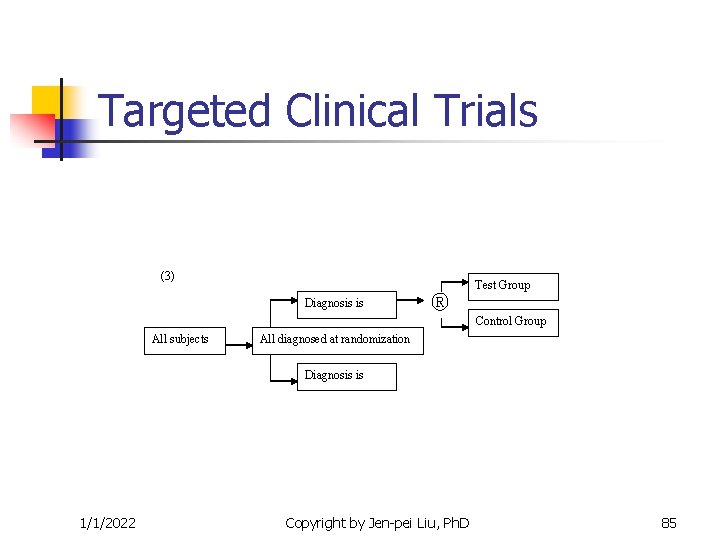
Targeted Clinical Trials (3) Diagnosis is ® Test Group Control Group All subjects All diagnosed at randomization Diagnosis is 1/1/2022 Copyright by Jen-pei Liu, Ph. D 85
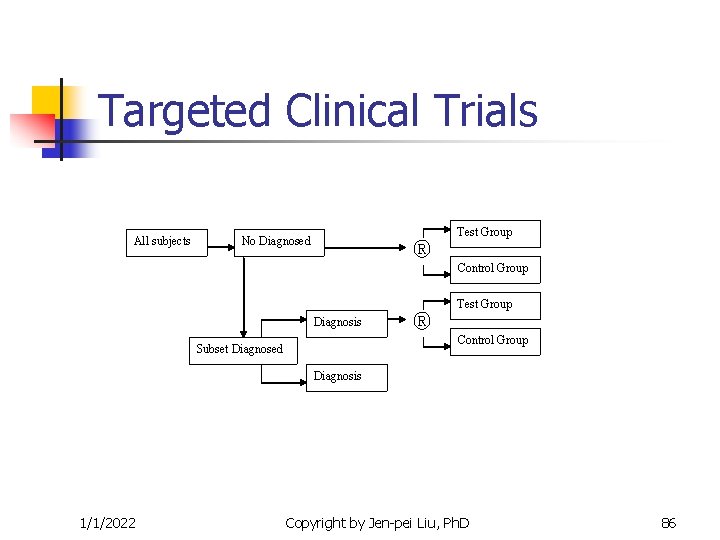
Targeted Clinical Trials All subjects No Diagnosed ® Test Group Control Group Diagnosis ® Test Group Control Group Subset Diagnosed Diagnosis 1/1/2022 Copyright by Jen-pei Liu, Ph. D 86
Multicenter Trials A multicenter study is a single study conducted under a common protocol, involving several centers (e. g. , clinics, practices, hospitals) where the data collected are intended to be analyzed as whole (as opposed to a post-hoc decision to combine data or results from separate studies). 1/1/2022 Copyright by Jen-pei Liu, Ph. D 87

Multicenter Trials n Goals n n n To accrue patients efficiently To provide a basis for generalization of its findings Centers n n n 1/1/2022 Single investigator at a single hospital Single investigator at more than one hospital A team of clinicians at several associated hospitals Cooperative groups→hospitals→investigator Countries →region→location→investigator Copyright by Jen-pei Liu, Ph. D 88

Examples n FACET International Study Group (NEJM 1997; 337: 1405 -11) A multinational and multicenter trial n Targeted population: 852 randomized patients n n 18 -70 years old Asthma >= 6 months Inhaled glucocorticoid >= 3 monyhs No. Centers: 71 in 9 countries 1/1/2022 Copyright by Jen-pei Liu, Ph. D 89
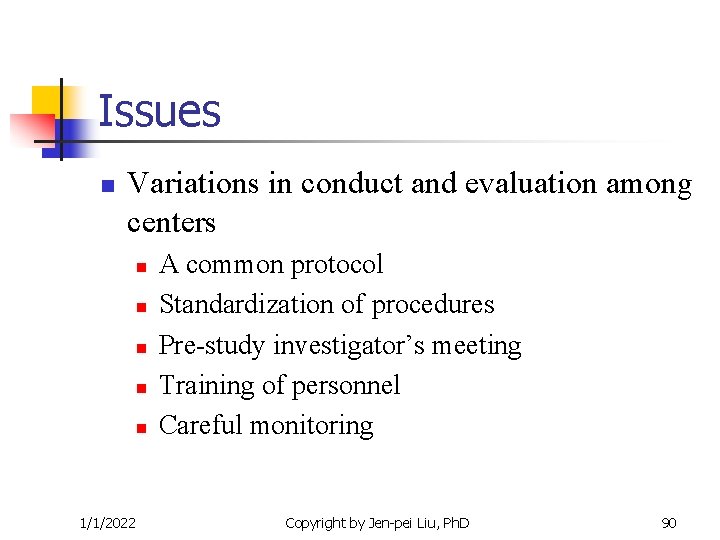
Issues n Variations in conduct and evaluation among centers n n n 1/1/2022 A common protocol Standardization of procedures Pre-study investigator’s meeting Training of personnel Careful monitoring Copyright by Jen-pei Liu, Ph. D 90
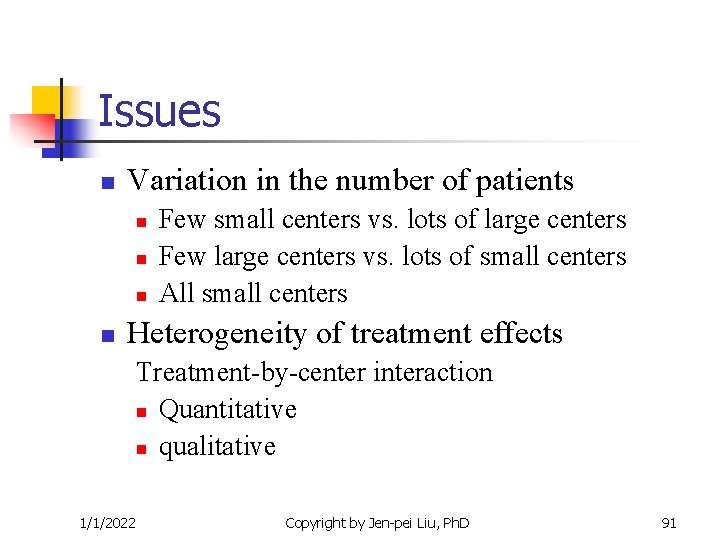
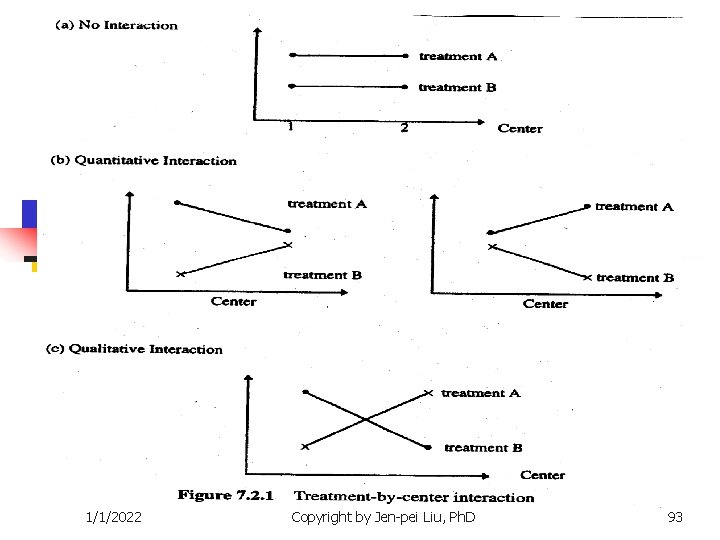
Issues n Variation in the number of patients n n Few small centers vs. lots of large centers Few large centers vs. lots of small centers All small centers Heterogeneity of treatment effects Treatment-by-center interaction n Quantitative n qualitative 1/1/2022 Copyright by Jen-pei Liu, Ph. D 91
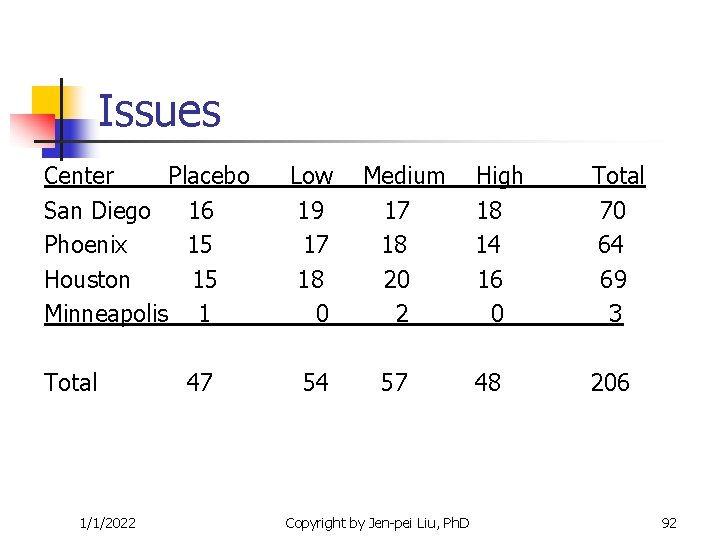
Issues Center Placebo San Diego 16 Phoenix 15 Houston 15 Minneapolis 1 Total 1/1/2022 47 Low 19 17 18 0 54 Medium 17 18 20 2 57 Copyright by Jen-pei Liu, Ph. D High 18 14 16 0 Total 70 64 69 3 48 206 92
1/1/2022 Copyright by Jen-pei Liu, Ph. D 93
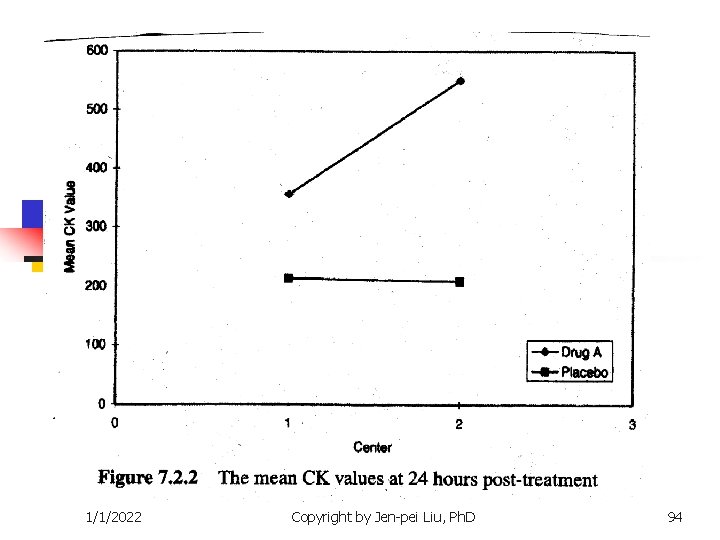
1/1/2022 Copyright by Jen-pei Liu, Ph. D 94

Issues n Fixed or random center effects n n n 1/1/2022 Recruit a minimal # of patients Expertise and experience of investigators Special equipments or facilities required by the study Not for comparisons among centers A classification factor and not a design factor Copyright by Jen-pei Liu, Ph. D 95

Superiority Trials The objective of the trial is to establish the efficacy by demonstrating that the test treatment is superior to n a concurrent placebo control n a concurrent active treatment control or n 1/1/2022 a dose-response relationship Copyright by Jen-pei Liu, Ph. D 96
Superiority Trials 1/1/2022 Copyright by Jen-pei Liu, Ph. D 97

Examples Canadian Beclomethasone Dipropoinate Salmethrol Xinafoate Study Group (NEJM, 1997; 337: 1659 -65) n Patient population 241 children, 6 -14 years old with stable asthma n Test treatment Long-acting β 2 -adrenergic-recepto agonist Salmethrol Xinafoate (50 ug twice daily) Active treatment concurrent control Glucocorticoid Beclomethasone (200 ug twice daily) Placebo concurrent. Copyright controlby Jen-pei Liu, Ph. D 1/1/2022 98

Concept of Equivalence n n To show that the efficacy of the new treatment is either as effective as or is no worse than the concurrent active control (standard therapy) Therapeutic areas n n Oncology Infectious diseases Bioequivalence Reasons Less toxic n Easier to administer n Substitution of an invasive procedure n Less expensive (cost-effectiveness) n Better quality of life 1/1/2022 Copyright by Jen-pei Liu, Ph. D n Generic drugs n 99

Two-sided equivalence-similarity n n The effect of the test treatments does not differ substantially in either direction from that of the control As compared to the concurrent active control The effect of the test treatment is not too low (the effect of the test treatment is not inferior) and The effect of the test treatment is not too high (the effect of the test treatment is not superior ) The effect of the test treatment should be within allowable equivalence limits of that of the control 1/1/2022 Copyright by Jen-pei Liu, Ph. D 100

One-sided equivalence-non-inferiority Therapeutic equivalence Because the test treatment offers some advantages over the control, the question is whether its effectiveness is within a specific tolerance of the control n One-sided problem the effect of the test treatment is not too low n 1/1/2022 Copyright by Jen-pei Liu, Ph. D 101
Example COBALT Investigators and Ware and Antman (NEJM 1997; 337: 1124 -30; 1159 -61) n Patient population 7169 patients with myocardial infarction n Objective To demonstrate that the 30 -day mortality of double-bolus alteplase (a bolus of 50 mg over 1 -3 minutes followed by 30 minutes later by a second bolus of 50 mg) is no worse than that of the accelerated infusion of 100 mg of alteplase. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 102
Statistical Formulations of Equivalence Only consider two treatment groups μT: the average effect of the test treatment μR: the average effect of the active control δL: the allowable lower equivalence limit (-) δU: the allowable upper equivalence limit (+) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 103
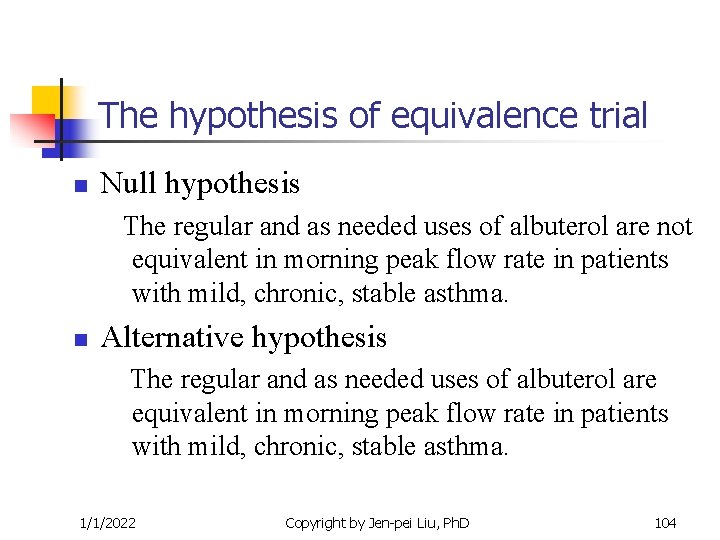
The hypothesis of equivalence trial n Null hypothesis The regular and as needed uses of albuterol are not equivalent in morning peak flow rate in patients with mild, chronic, stable asthma. n Alternative hypothesis The regular and as needed uses of albuterol are equivalent in morning peak flow rate in patients with mild, chronic, stable asthma. 1/1/2022 Copyright by Jen-pei Liu, Ph. D 104
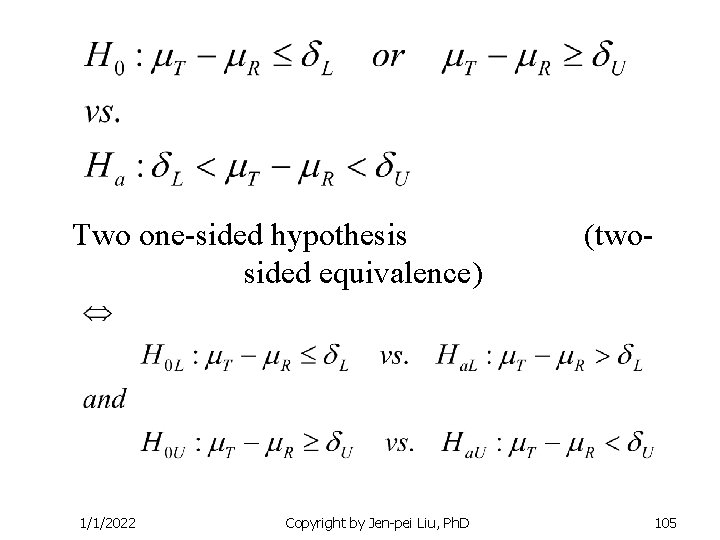
Two one-sided hypothesis sided equivalence) 1/1/2022 Copyright by Jen-pei Liu, Ph. D (two- 105
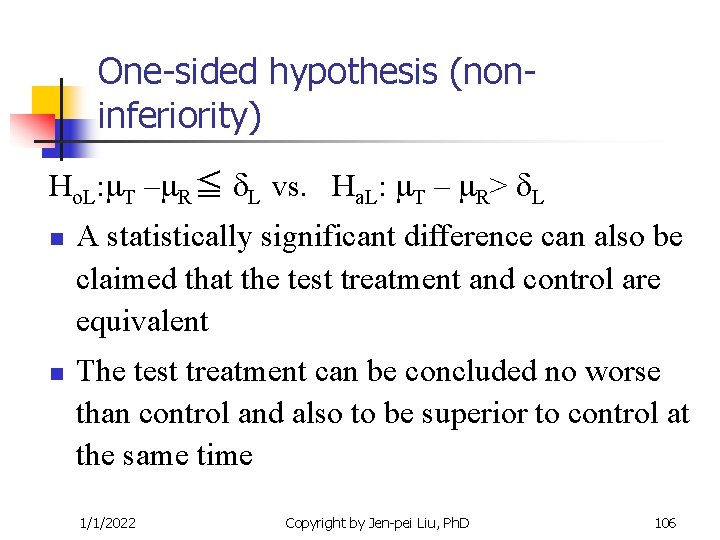
One-sided hypothesis (noninferiority) Ho. L: T – R≦ L vs. Ha. L: T – R> L n n A statistically significant difference can also be claimed that the test treatment and control are equivalent The test treatment can be concluded no worse than control and also to be superior to control at the same time 1/1/2022 Copyright by Jen-pei Liu, Ph. D 106
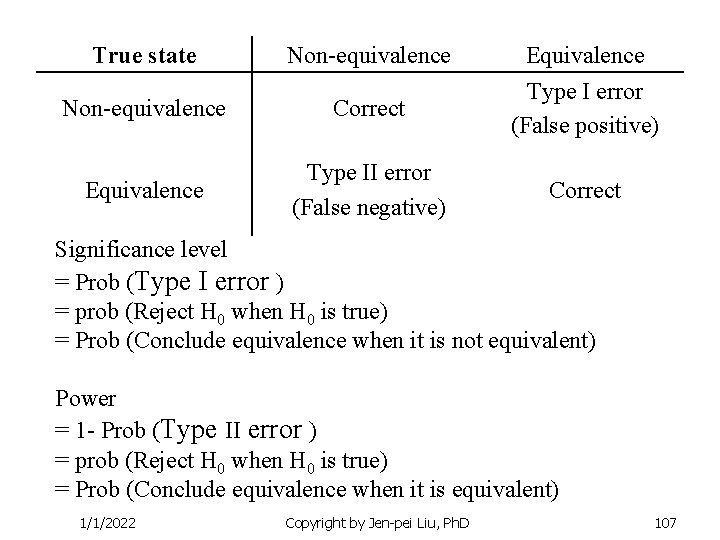
True state Non-equivalence Correct Equivalence Type II error (False negative) Equivalence Type I error (False positive) Correct Significance level = Prob (Type I error ) = prob (Reject H 0 when H 0 is true) = Prob (Conclude equivalence when it is not equivalent) Power = 1 - Prob (Type II error ) = prob (Reject H 0 when H 0 is true) = Prob (Conclude equivalence when it is equivalent) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 107
Issues of equivalence or non-inferiority trials n n Lack of internal validity without concurrent placebo control Determination of equivalence margins Largest difference that is clinically acceptable n Equivalence trials Both upper and lower margins n Non-inferiority trials Only the lower margin n COBALT a difference of 0. 4% in 30 -day mortality rate between double bolus and accelerated bolus 1/1/2022 Copyright by Jen-pei Liu, Ph. D 108

Issues of Active Control Trials n n n Efficacy of a treatment should be established against placebo Equivalence or non-inferiority of test treatment to the active control both superior to placebo both inferior to placebo The efficacy of the active control in the relevant indication has been clearly established and quantified in well-design and welldocumented superiority trials and that can be reliably expected to exhibit similar efficacy in the contemplated active control trial 1/1/2022 Copyright by Jen-pei Liu, Ph. D 109

Issues of Active Control Trials n n n Same design features n Inclusion / exclusion criteria n Dose n Primary endpoints Objective of equivalence or non-inferiority must be stated in the protocols with the equivalence limits Inclusion of a concurrent placebo control for interval validity Superiority of active concurrent control to placebo Equivalence of the test treatment to active concurrent control 1/1/2022 Copyright by Jen-pei Liu, Ph. D 110
Determination of Equivalence Limit n n n Largest difference which can be judged as being clinically acceptable Smaller than the relative efficacy of active concurrent control compared to placebo established in well-designed and welldocumented superior trial Equivalence trials Both upper and lower margins Non-inferiority trials Only the lower margin Must be stated in the protocol Should be clinical justification for the choice of equivalence limits 1/1/2022 Copyright by Jen-pei Liu, Ph. D 111
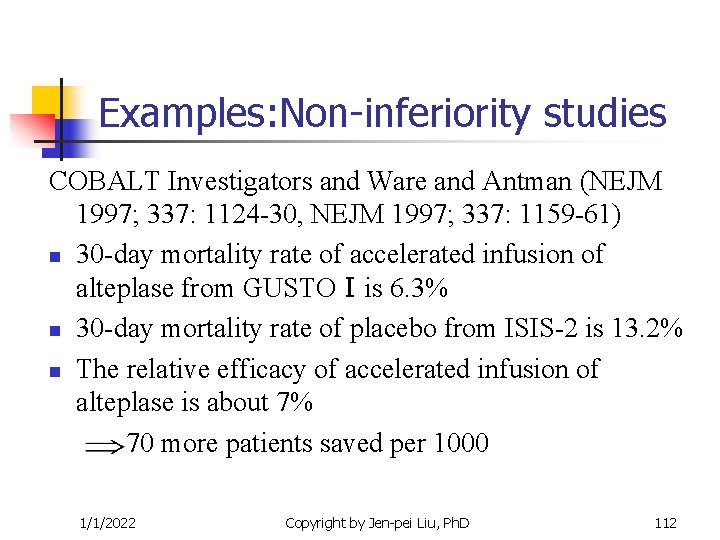
Examples: Non-inferiority studies COBALT Investigators and Ware and Antman (NEJM 1997; 337: 1124 -30, NEJM 1997; 337: 1159 -61) n 30 -day mortality rate of accelerated infusion of alteplase from GUSTOⅠis 6. 3% n 30 -day mortality rate of placebo from ISIS-2 is 13. 2% n The relative efficacy of accelerated infusion of alteplase is about 7% 70 more patients saved per 1000 1/1/2022 Copyright by Jen-pei Liu, Ph. D 112
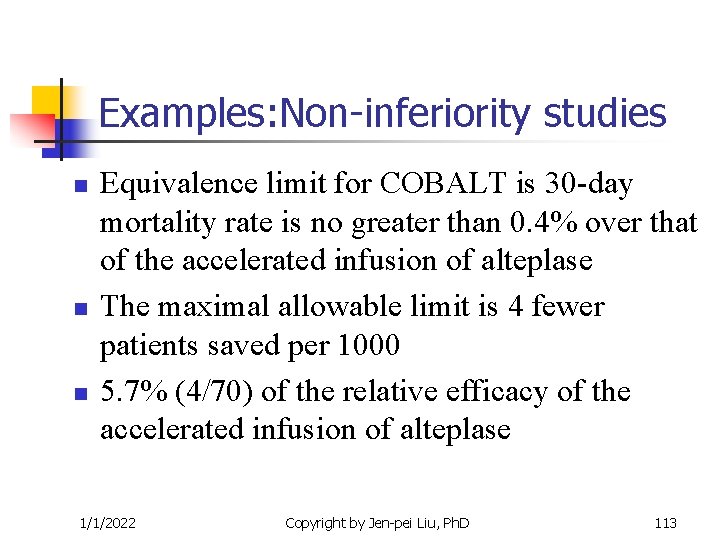
Examples: Non-inferiority studies n n n Equivalence limit for COBALT is 30 -day mortality rate is no greater than 0. 4% over that of the accelerated infusion of alteplase The maximal allowable limit is 4 fewer patients saved per 1000 5. 7% (4/70) of the relative efficacy of the accelerated infusion of alteplase 1/1/2022 Copyright by Jen-pei Liu, Ph. D 113
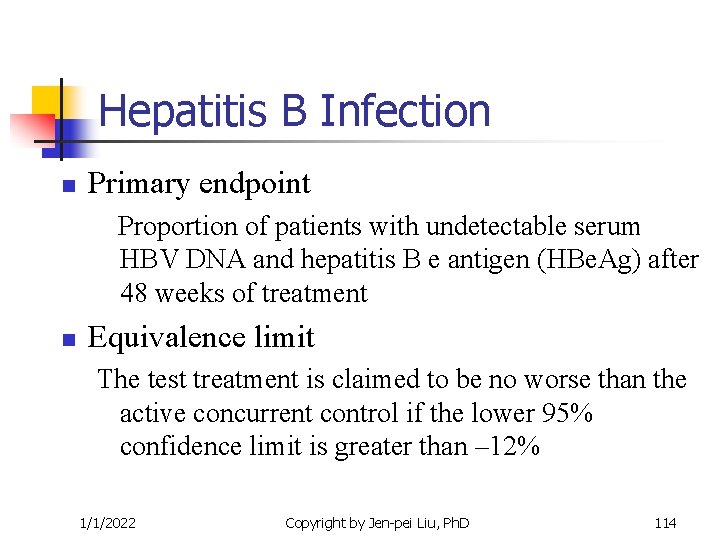
Hepatitis B Infection n Primary endpoint Proportion of patients with undetectable serum HBV DNA and hepatitis B e antigen (HBe. Ag) after 48 weeks of treatment n Equivalence limit The test treatment is claimed to be no worse than the active concurrent control if the lower 95% confidence limit is greater than – 12% 1/1/2022 Copyright by Jen-pei Liu, Ph. D 114

Hepatitis B Infection n The response rate for the active concurrent control for sample size determination is assumed to be 20% The placebo response rate is 10% In other word, the response rate of the test treatment can be as low as 8% to be declared to be non-inferior to the active concurrent control 1/1/2022 Copyright by Jen-pei Liu, Ph. D 115
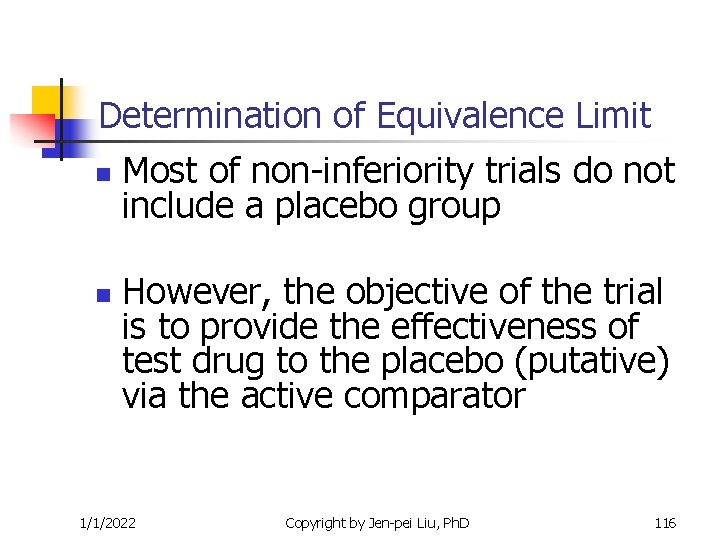
Determination of Equivalence Limit n n Most of non-inferiority trials do not include a placebo group However, the objective of the trial is to provide the effectiveness of test drug to the placebo (putative) via the active comparator 1/1/2022 Copyright by Jen-pei Liu, Ph. D 116

Determination of Equivalence Limit n n The effectiveness of the active comparator have been approved because of its superiority over placebo The non-inferiority margin should be formulated so that the test drug can preserve the effectiveness of the active comparator 1/1/2022 Copyright by Jen-pei Liu, Ph. D 117
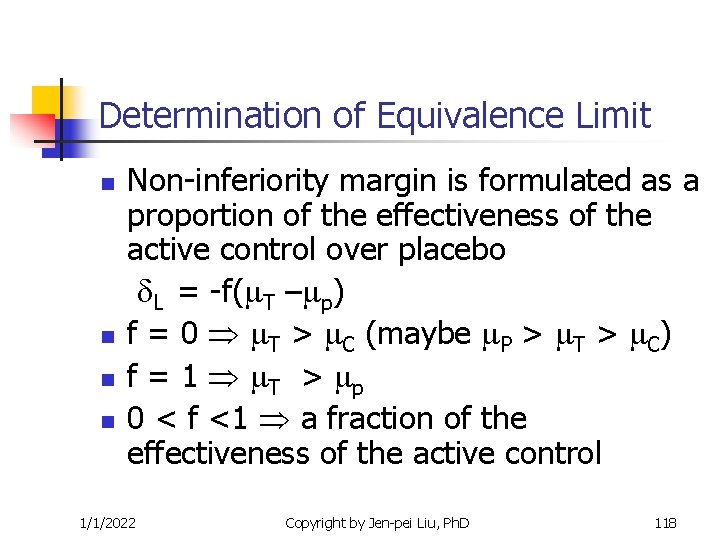
Determination of Equivalence Limit n n Non-inferiority margin is formulated as a proportion of the effectiveness of the active control over placebo L = -f( T – p) f = 0 T > C (maybe P > T > C) f = 1 T > p 0 < f <1 a fraction of the effectiveness of the active control 1/1/2022 Copyright by Jen-pei Liu, Ph. D 118
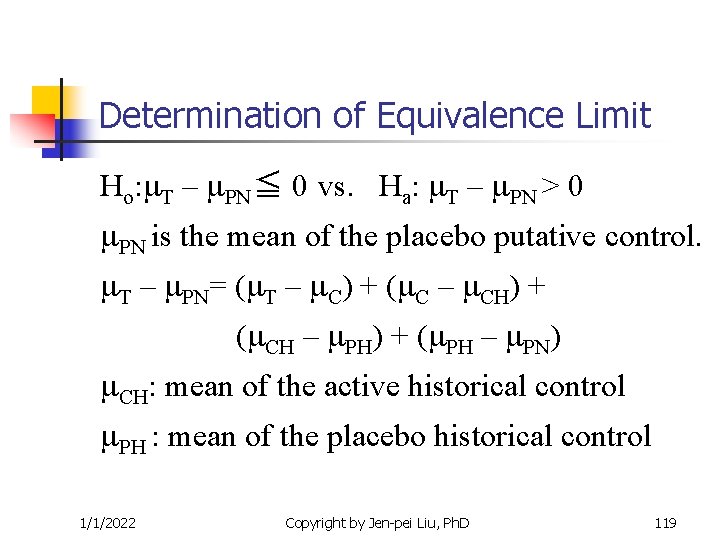
Determination of Equivalence Limit Ho: T – PN≦ 0 vs. Ha: T – PN > 0 PN is the mean of the placebo putative control. T – PN= ( T – C) + ( C – CH) + ( CH – PH) + ( PH – PN) CH: mean of the active historical control PH : mean of the placebo historical control 1/1/2022 Copyright by Jen-pei Liu, Ph. D 119
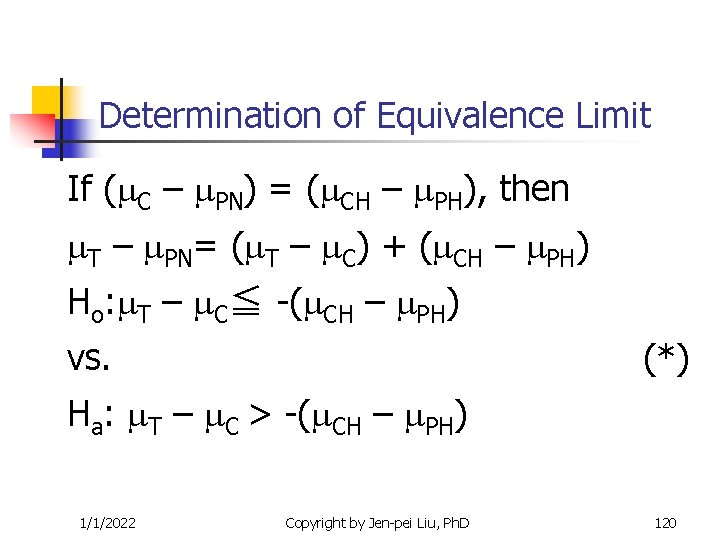
Determination of Equivalence Limit If ( C – PN) = ( CH – PH), then T – PN= ( T – C) + ( CH – PH) Ho: T – C≦ -( CH – PH) vs. (*) Ha: T – C > -( CH – PH) 1/1/2022 Copyright by Jen-pei Liu, Ph. D 120
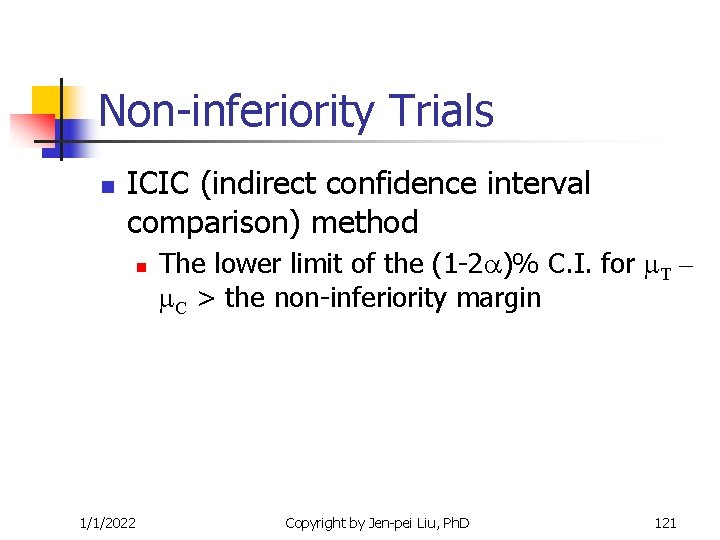
Non-inferiority Trials n ICIC (indirect confidence interval comparison) method n 1/1/2022 The lower limit of the (1 -2 )% C. I. for T – C > the non-inferiority margin Copyright by Jen-pei Liu, Ph. D 121
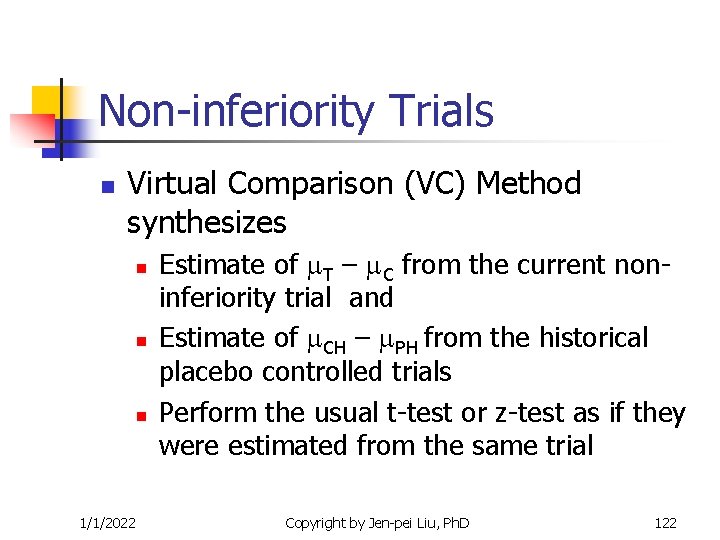
Non-inferiority Trials n Virtual Comparison (VC) Method synthesizes n n n 1/1/2022 Estimate of T – C from the current noninferiority trial and Estimate of CH – PH from the historical placebo controlled trials Perform the usual t-test or z-test as if they were estimated from the same trial Copyright by Jen-pei Liu, Ph. D 122

Non-inferiority Trials n n Constancy assumption – the effectiveness of the active control in the current non-inferiority trial is the same as that obtained from its historical placebo controlled trial VC – inflation of type I error if constancy assumption is not met ICIC is conservative if constancy assumption is satisfied ICIC is liberal if the effectiveness of the active control in the current non-inferiority trial is less than that of historical placebo controlled trial 1/1/2022 Copyright by Jen-pei Liu, Ph. D 123
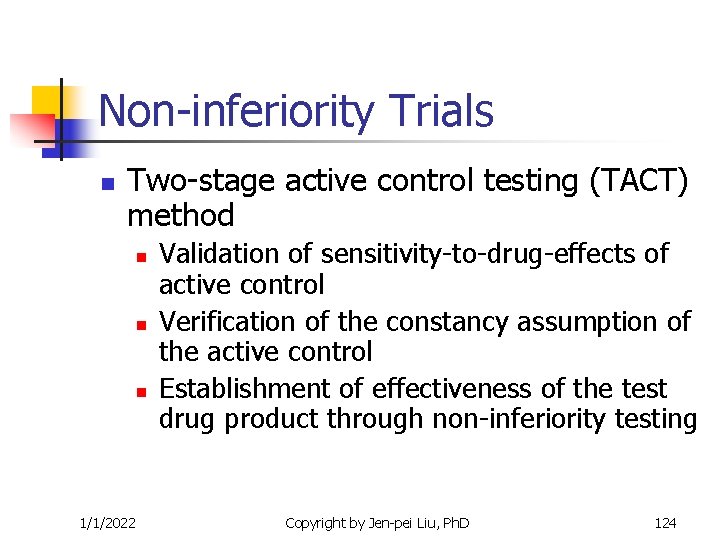
Non-inferiority Trials n Two-stage active control testing (TACT) method n n n 1/1/2022 Validation of sensitivity-to-drug-effects of active control Verification of the constancy assumption of the active control Establishment of effectiveness of the test drug product through non-inferiority testing Copyright by Jen-pei Liu, Ph. D 124
Group Sequential Trials A group sequential trials allows to evaluate the efficacy and safety of test treatment by means of interim analyses during the study for possible early termination based on convincing evidence of either benefit or harm before its scheduled completion n Description of pre-planned interim analyses in the protocol with adjustment of p-values n Documentation of everything n Independent data monitoring committee 1/1/2022 Copyright by Jen-pei Liu, Ph. D 125
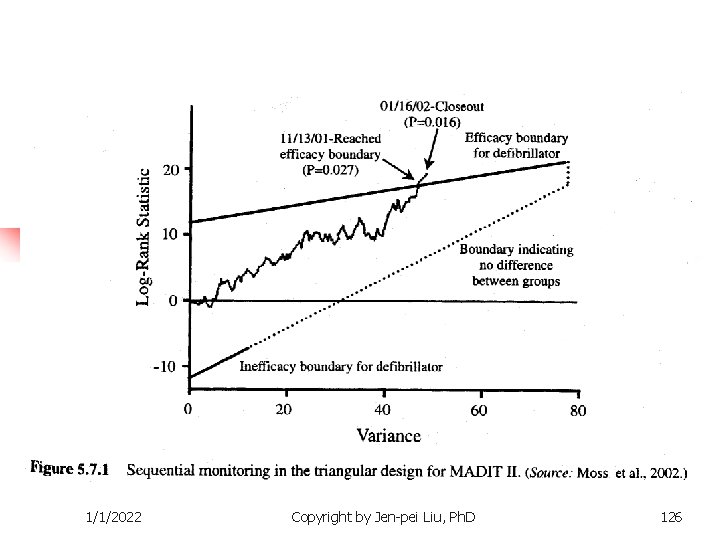
1/1/2022 Copyright by Jen-pei Liu, Ph. D 126
- Slides: 126