Static Electricity Unit 3 Physics Electricity Lesson 1


















- Slides: 28

Static Electricity Unit 3: Physics - Electricity Lesson 1

Do not copy • Staticy clothing and electric shocks felt when touching doorknobs on are caused by electric charges.

Electric Charges • Electric charges are charged particles that exert an electric force on each other. – (Do not copy) Charged particles are very small but when they are present in large enough quantities they can produce sparks just large enough to feel or large enough to kill.

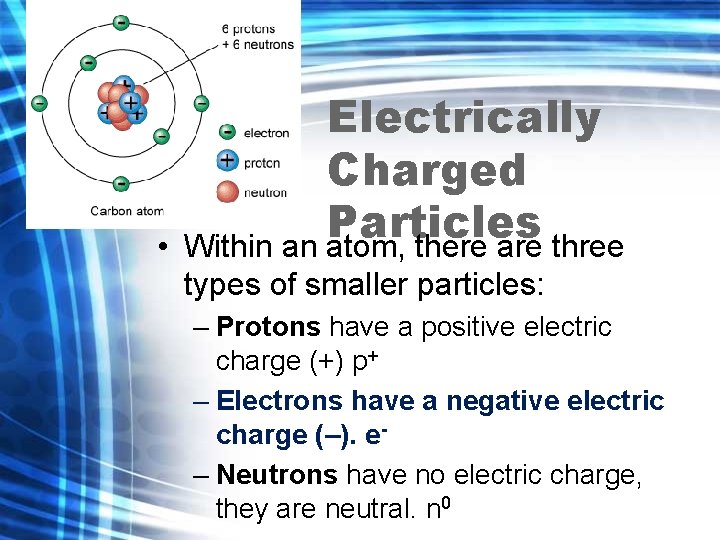
Electrically Charged Particles (Review) • An element is a pure substance that cannot be broken down into simpler substances. • An element is made up of tiny particles called atoms.

• Electrically Charged Particles Within an atom, there are three types of smaller particles: – Protons have a positive electric charge (+) p+ – Electrons have a negative electric charge (–). e– Neutrons have no electric charge, they are neutral. n 0

Electrically Charged Particles • The protons and neutrons are in the nucleus at the centre of the atom. • The electrons are outside the nucleus

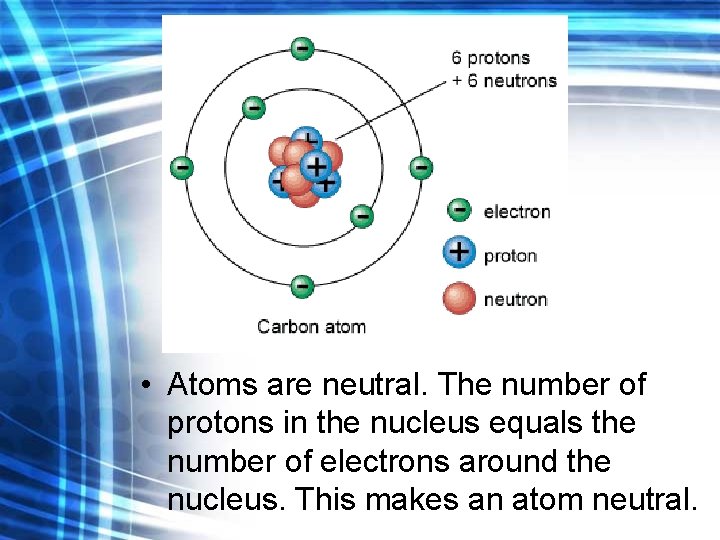
• Atoms are neutral. The number of protons in the nucleus equals the number of electrons around the nucleus. This makes an atom neutral.

• If an atom does not have an equal number of protons and electrons, it has an electric charge. – Electric charge – A form of charge, either positive or negative, that exerts an electric force.

Types of charges on objects • Neutral Object – an object that has equal numbers of protons and electrons • Negatively charged object – an object that has more electrons than protons • Positively charged object – an object that has fewer electrons than protons.

Static Charges • Objects can become charged when electrons move from one object to another. • The electric charge that builds up on the surface of the object is called a static charge or static electricity.

Static Charges • The charges are “static” because they remain very nearly fixed in one location on the surface of the object until they are given a path to escape

• Electrostatics is the study of electric charges

Friction and the Movement of Electrons

Friction • All solid materials are charged by the transfer of electrons. – One common cause of electron transfer is friction.

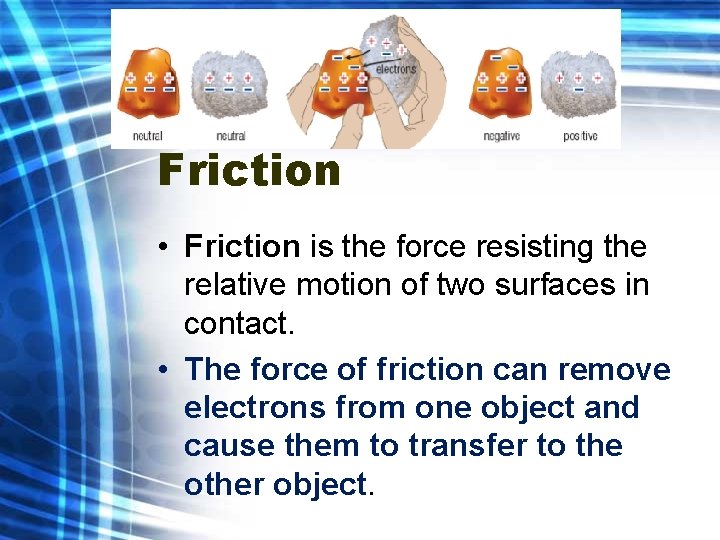
Friction • Friction is the force resisting the relative motion of two surfaces in contact. • The force of friction can remove electrons from one object and cause them to transfer to the other object.

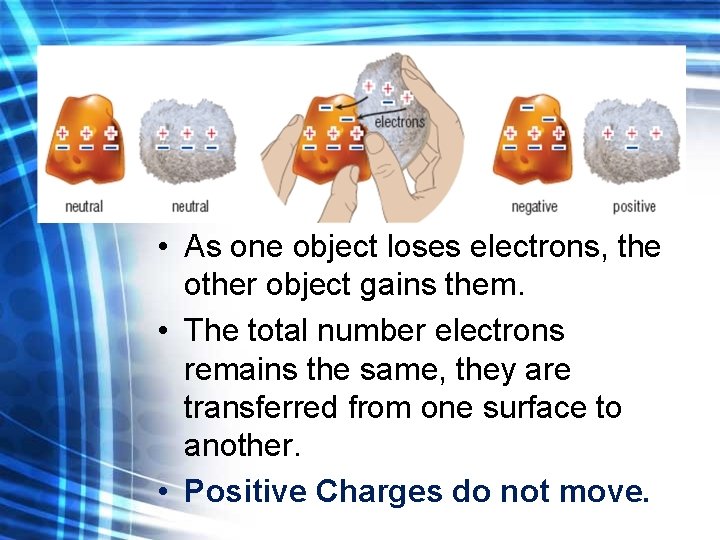
Friction • As one object loses electrons, the other object gains them. • The total number electrons remains the same, they are transferred from one surface to another. • Positive Charges do not move.

(don’t copy) • For any charging procedure, it’s important to keep in mind that new electric charges are not being created. The electrons in each object are just being rearranged within the object or transferred to another object.

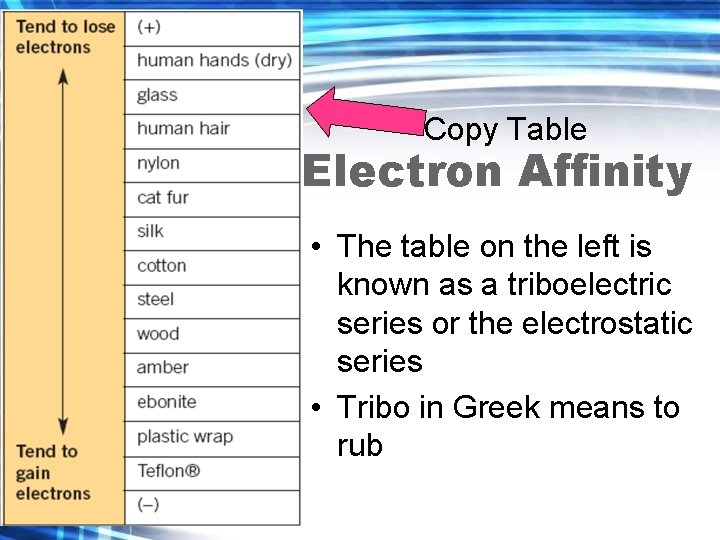
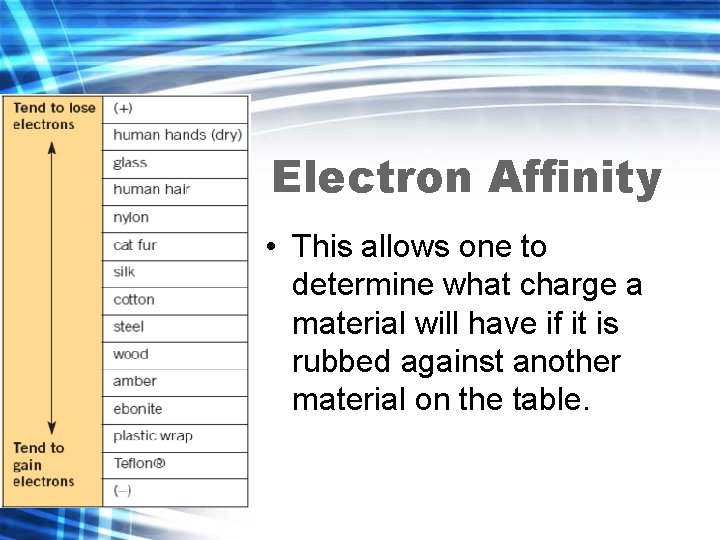
Electron Affinity and the Electrostatic Series • Different substances have different abilities to hold on to electrons. The tendency of a substance to hold on to the electrons is called electron affinity.

Electron Affinity and the Electrostatic Series • The electrostatic series lists a series of selected materials in order of their electron affinity. • The higher the material is in the list, the greater the tendency for that material to lose electrons.

Copy Table Electron Affinity • The table on the left is known as a triboelectric series or the electrostatic series • Tribo in Greek means to rub

Electron Affinity • This allows one to determine what charge a material will have if it is rubbed against another material on the table.

Do not copy • There can be a slightly different order for materials such as fur or wood depending on which type of animal the fur is from and which type of tree the wood is from

Laws of Attraction and Repulsion

Law of attraction. • Particles with opposite charges attract each other.

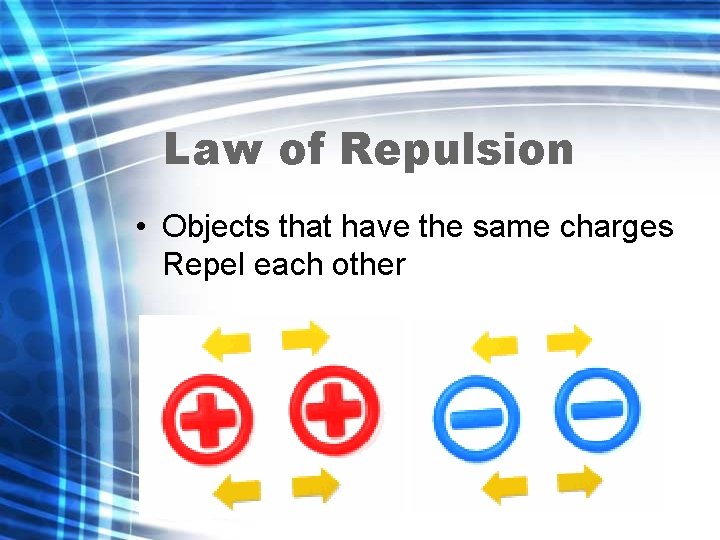
Law of Repulsion • Objects that have the same charges Repel each other

note • when a charged object is brought near a neutral object it will attract it.

Questions • 1. Where are electrons in the atom? K (1) • 2. What does “static” mean in “static electricity”? . • 3. What happens when two objects made out of different materials are rubbed together? • 4. What term describes an atom’s tendency to hold on to electrons? K (1)

Questions • 5. In each of the following pairs, state which one is more likely to give up electrons? I (3) • (a) wood or human hair • (b) plastic wrap or steel • (c) cotton or silk. • 6. (a) What does the law of attraction state? • (b) What does the law of repulsion state? K (2)