STATIC ELECTRICITY ATOMIC STRUCTURE Summary of Subatomic Particles

STATIC ELECTRICITY

ATOMIC STRUCTURE Summary of Subatomic Particles Proton Neutron Electron In nucleus Tightly Bound Positive Charge Massive In nucleus Tightly Bound No Charge Massive Outside nucleus Weakly Bound Negative Charge Not very massive

CHARGED & NEUTRAL OBJECTS • Charged objects occur due to an imbalance of protons & neutrons. • Negatively charged objects- have more electrons than protons. • Positively charged objects- have more protons than electrons. • Neutral objects-have equal numbers of protons and electrons

CONDUCTORS & INSULATORS • Conductor-a material that allows charges to move about easily. • Metals • It is easy to remove electrons from metal atoms, because of this metallic substances allow for electrons to flow freely throughout the object as a whole. • Insulator-A material through which a charge will not move easily. • Glass • Plastic (most) • Dry wood • Cloth • Dry air
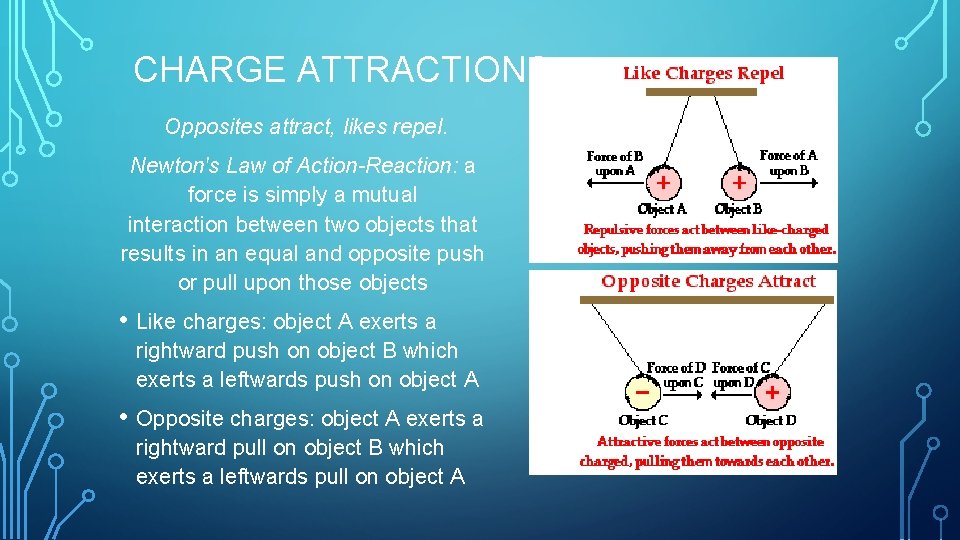
CHARGE ATTRACTIONS Opposites attract, likes repel. Newton's Law of Action-Reaction: a force is simply a mutual interaction between two objects that results in an equal and opposite push or pull upon those objects • Like charges: object A exerts a rightward push on object B which exerts a leftwards push on object A • Opposite charges: object A exerts a rightward pull on object B which exerts a leftwards pull on object A

SUMMARY OF ELECTRIC FORCE • There are two types of electric charge: positive & negative • Charges exert forces on other charges at a distance • The force is stronger when the charges are closer together. • Like charges repel, opposite charges attract
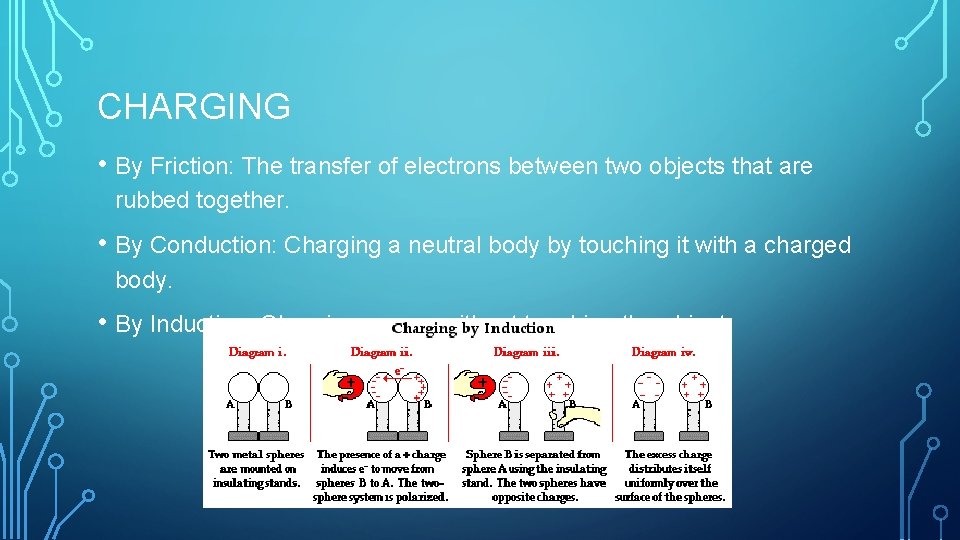
CHARGING • By Friction: The transfer of electrons between two objects that are rubbed together. • By Conduction: Charging a neutral body by touching it with a charged body. • By Induction: Charging occurs without touching the object.
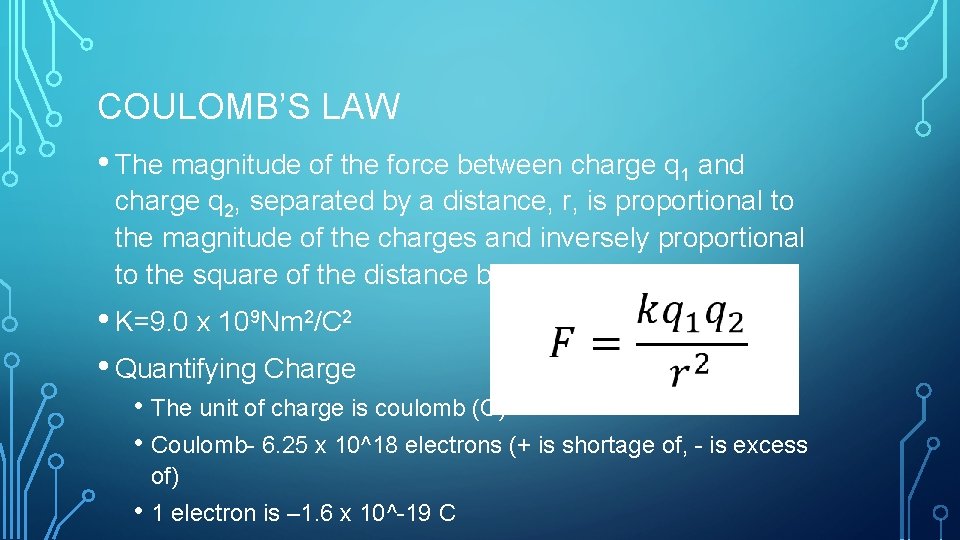
COULOMB’S LAW • The magnitude of the force between charge q 1 and charge q 2, separated by a distance, r, is proportional to the magnitude of the charges and inversely proportional to the square of the distance between them. • K=9. 0 x 109 Nm 2/C 2 • Quantifying Charge • The unit of charge is coulomb (C) • Coulomb- 6. 25 x 10^18 electrons (+ is shortage of, - is excess of) • 1 electron is – 1. 6 x 10^-19 C

PRACTICE PROBLEM • Sphere A, with a charge of +6. 0 u. C, is located near another charged sphere, B. Sphere B has a charge of -3. 0 u. C and is located 4. 0 cm to the right of A. • • What is the force of sphere B on sphere A? A third sphere, C, with a +1. 5 u. C charge, is added to the configuration. If it is located 3 cm beneath A, what is the new net force on sphere A?

3/23 OPENER Two charges are 40 cm apart. Charge A is 4 u. C and charge B is 8 u. C, what is the Force of B on A? k=9 x 10^9 Nm 2/C 2

TRIBOELECTRIC SERIES Different materials have different affinities for electrons. The triboelectric series can be used to determine the charge of materials when paired. Materials higher in the series have a greater affinity for electrons, therefore, when paired, you can compare materials order in the series to determine which material will have a more negative charge. *You may need to do some research to find triboelectric series that has your materials in it.

ELECTROSCOPE An electroscope is a device used for detecting charges. • When neutral the leaves of aluminum hang loosely, almost touching. • When charged with a negatively charged material the leaves spread further apart. • When charged with a positively charged material the leaves spread slightly apart.
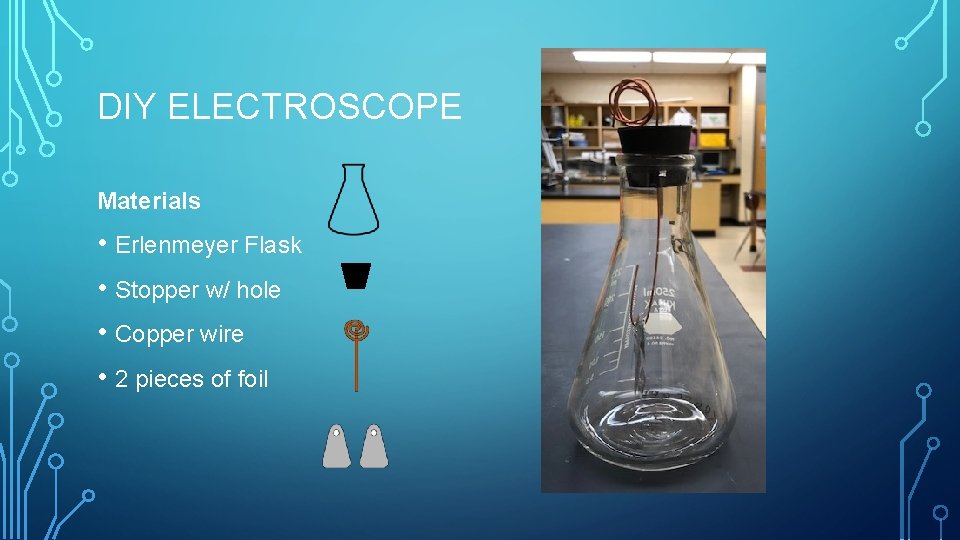
DIY ELECTROSCOPE Materials • Erlenmeyer Flask • Stopper w/ hole • Copper wire • 2 pieces of foil
- Slides: 13