STATIC ELECTRICITY A Particle Model of Electricity OUTCOME






- Slides: 30

STATIC ELECTRICITY: A Particle Model of Electricity

OUTCOME QUESTION(S): S 1 -3 -04: How does the Atomic Model help to explain static electricity? Vocabulary & Concepts Neutral Conservation of Charge Insulator Conductor Polarization

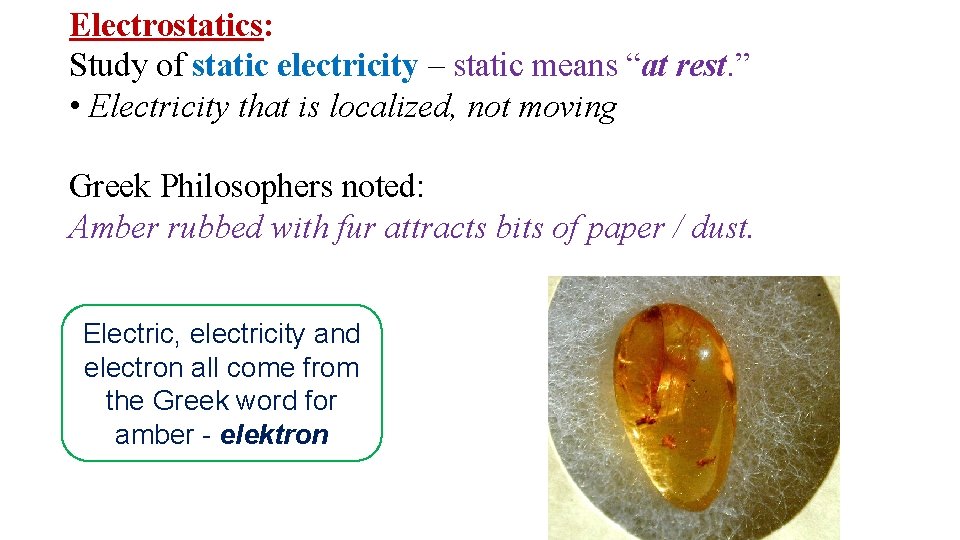
Electrostatics: Study of static electricity – static means “at rest. ” • Electricity that is localized, not moving Greek Philosophers noted: Amber rubbed with fur attracts bits of paper / dust. Electric, electricity and electron all come from the Greek word for amber - elektron

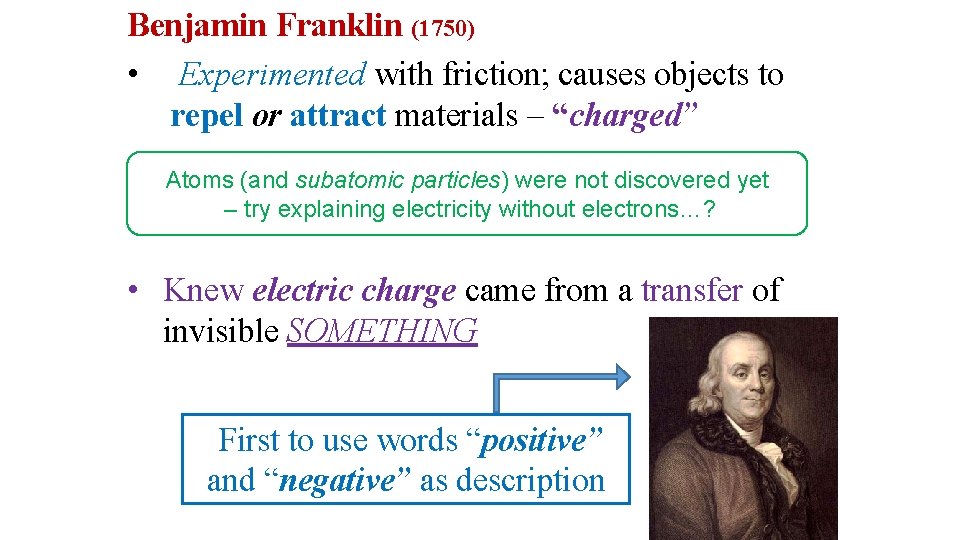
Benjamin Franklin (1750) • Experimented with friction; causes objects to repel or attract materials – “charged” Atoms (and subatomic particles) were not discovered yet – try explaining electricity without electrons…? • Knew electric charge came from a transfer of invisible SOMETHING First to use words “positive” and “negative” as description

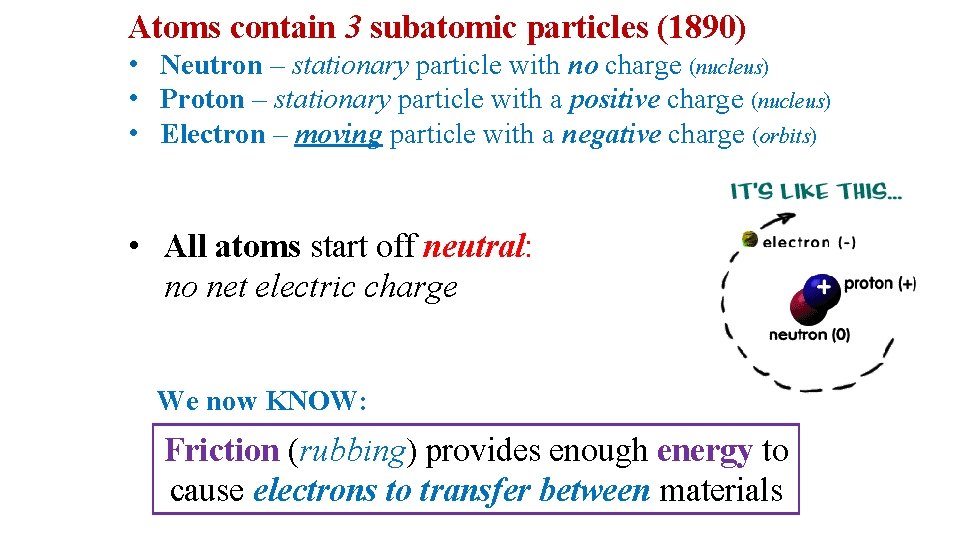
Atoms contain 3 subatomic particles (1890) • Neutron – stationary particle with no charge (nucleus) • Proton – stationary particle with a positive charge (nucleus) • Electron – moving particle with a negative charge (orbits) • All atoms start off neutral: no net electric charge We now KNOW: Friction (rubbing) provides enough energy to cause electrons to transfer between materials

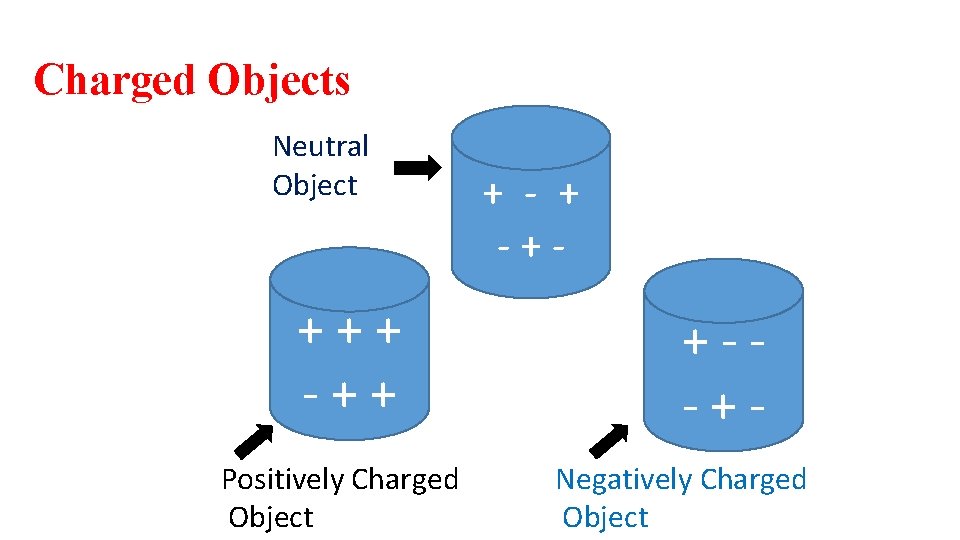
Charged Objects Neutral Object +++ -++ Positively Charged Object + -+- +--+Negatively Charged Object

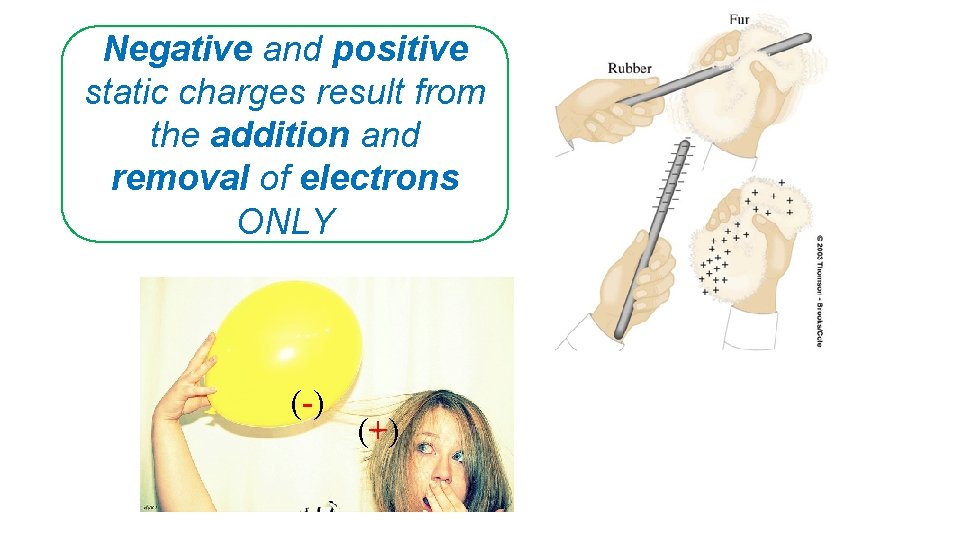
Negative and positive static charges result from the addition and removal of electrons ONLY (-) (+)

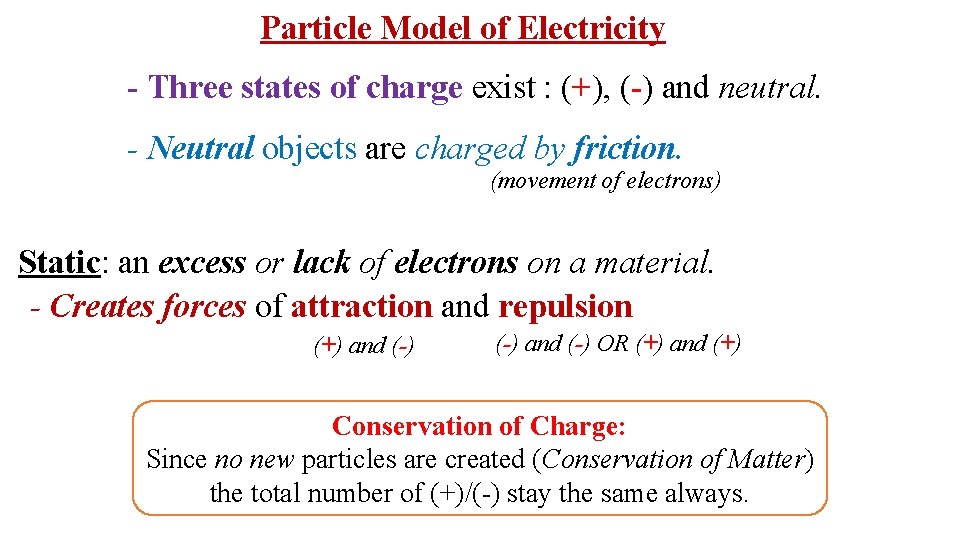
Particle Model of Electricity - Three states of charge exist : (+), (-) and neutral. - Neutral objects are charged by friction. (movement of electrons) Static: an excess or lack of electrons on a material. - Creates forces of attraction and repulsion (+) and (-) OR (+) and (+) Conservation of Charge: Since no new particles are created (Conservation of Matter) the total number of (+)/(-) stay the same always.

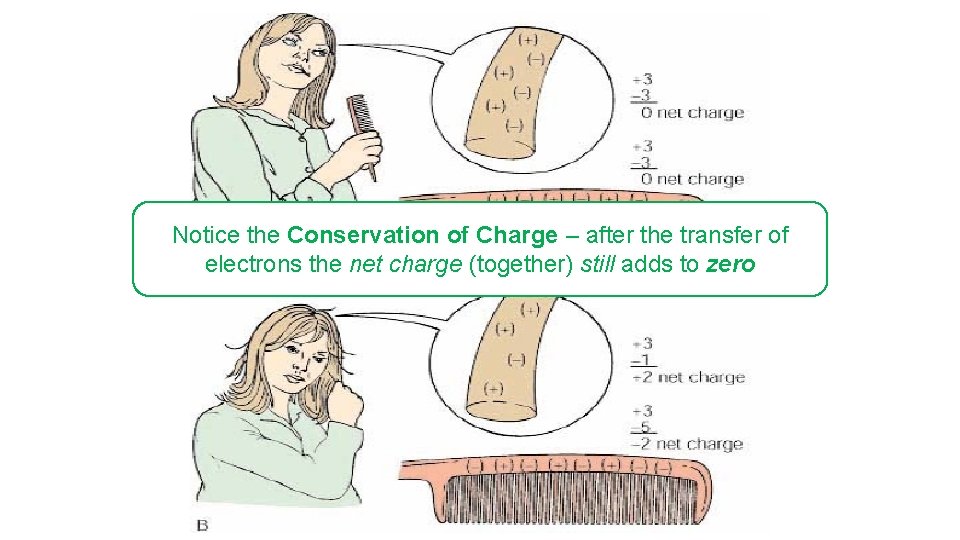
Notice the Conservation of Charge – after the transfer of electrons the net charge (together) still adds to zero

When rubbed together, materials have a different strength of attraction for the electrons Most Positive (+) Easily lose electrons Human Skin Fur Glass Human Hair Wool Silk Paper +++ + Cotton Easily gain electrons Rubber Copper Silver Polyester Plastic Most Negative (-) ---

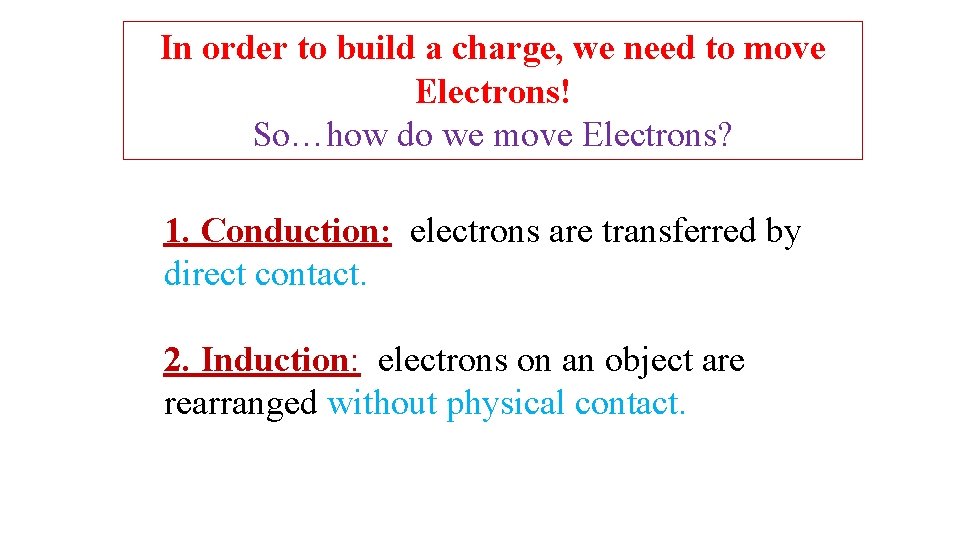
In order to build a charge, we need to move Electrons! So…how do we move Electrons? 1. Conduction: electrons are transferred by direct contact. 2. Induction: electrons on an object are rearranged without physical contact.

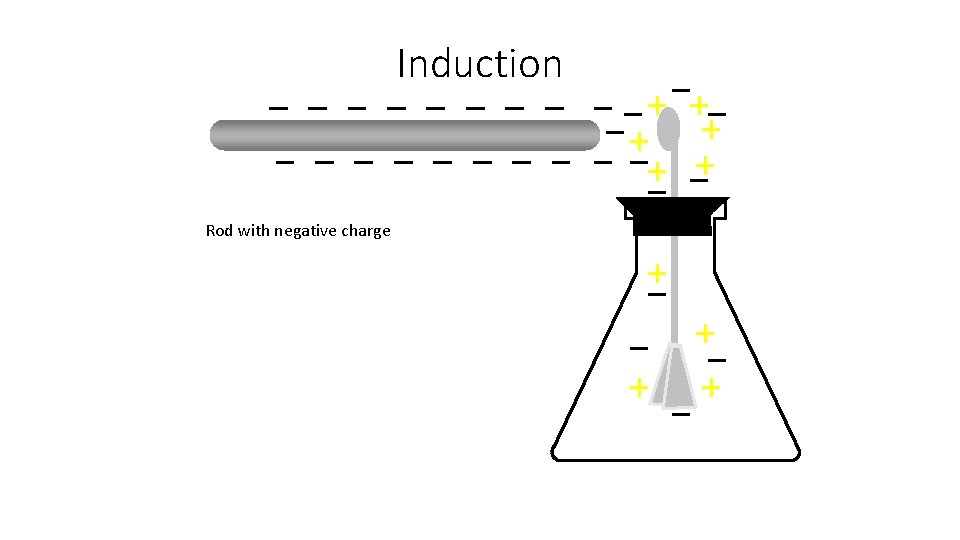
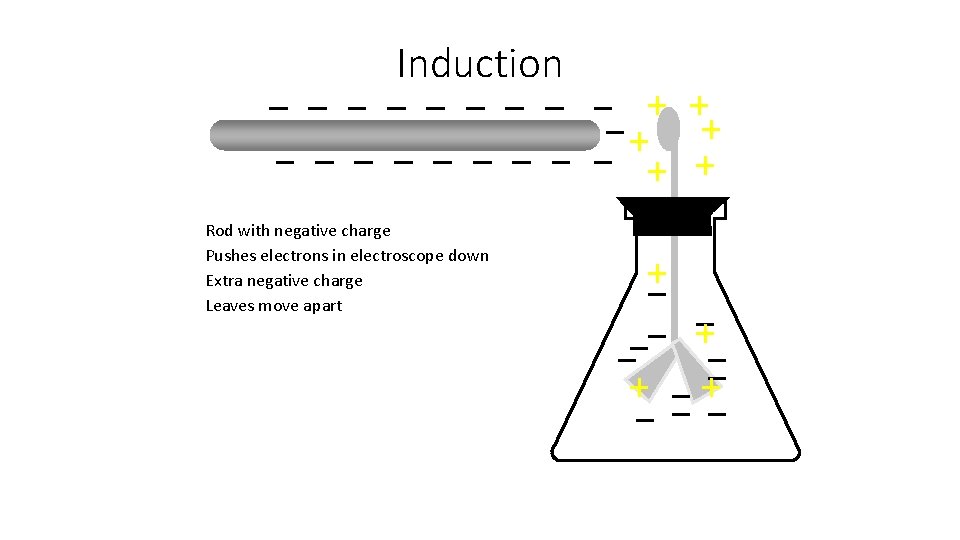
Induction Rod with negative charge

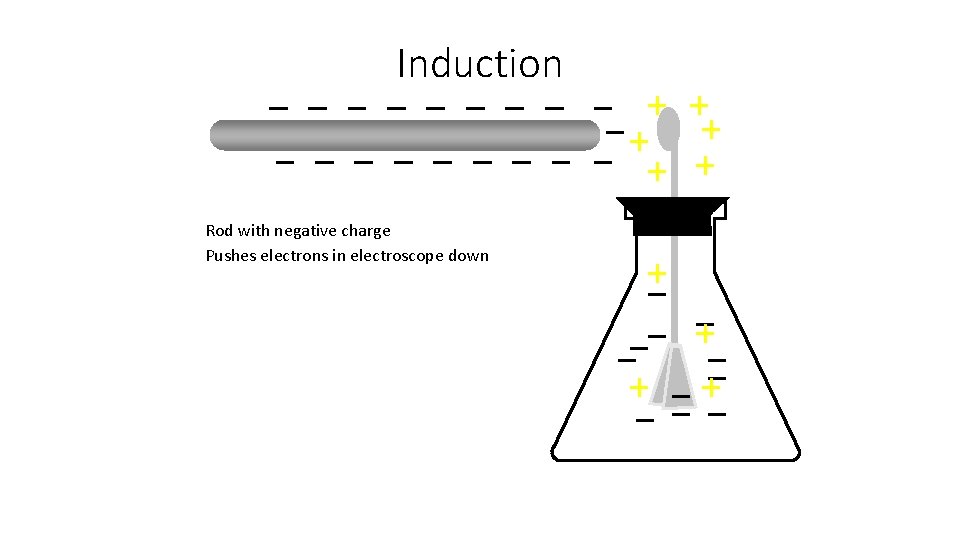
Induction Rod with negative charge Pushes electrons in electroscope down

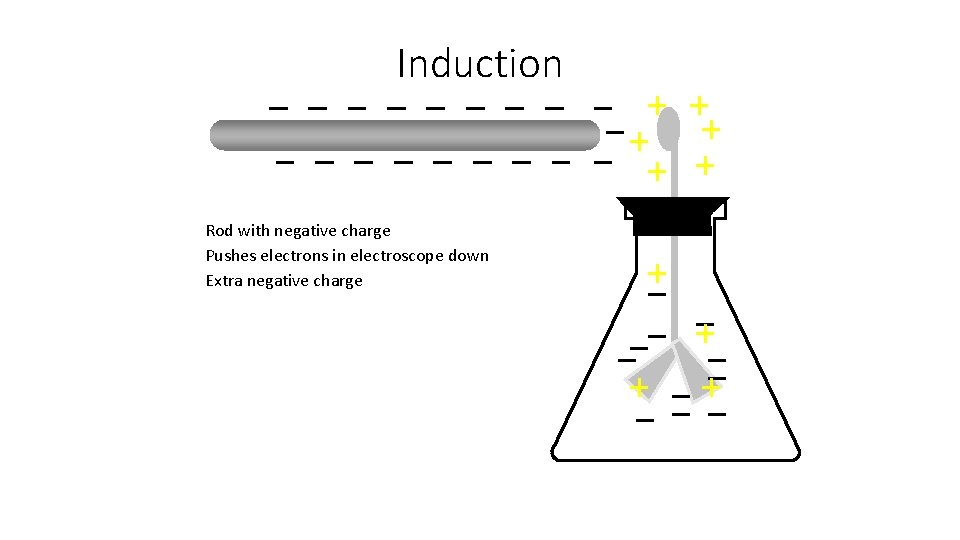
Induction Rod with negative charge Pushes electrons in electroscope down Extra negative charge

Induction Rod with negative charge Pushes electrons in electroscope down Extra negative charge Leaves move apart

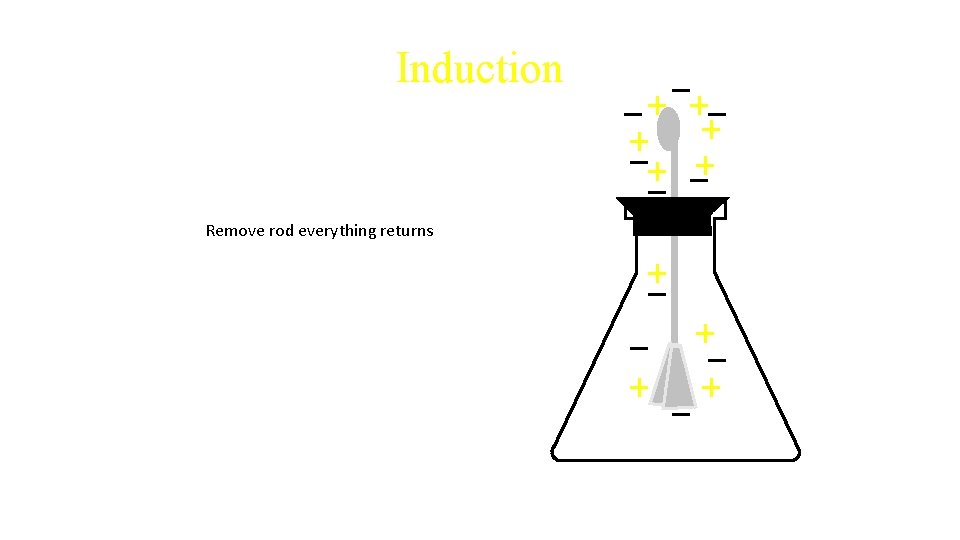
Induction Remove rod everything returns

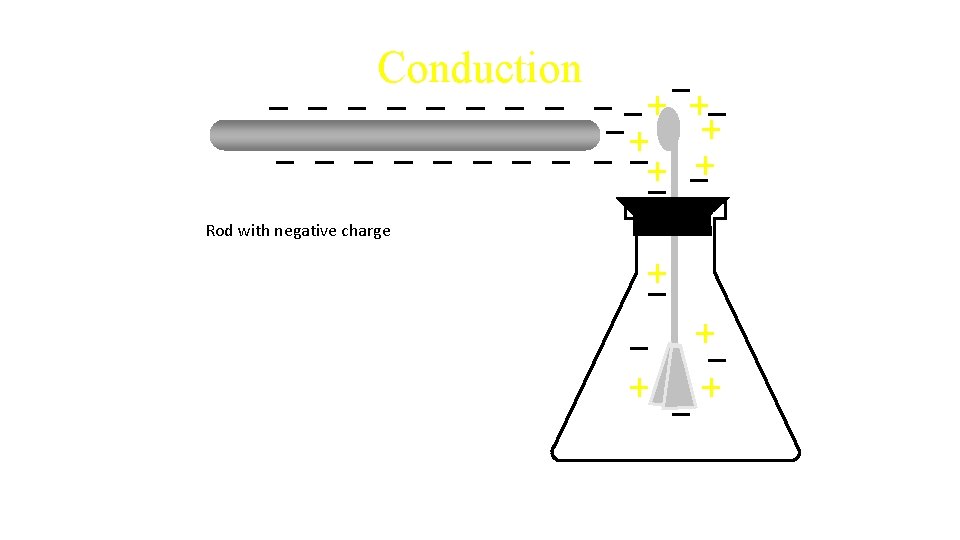
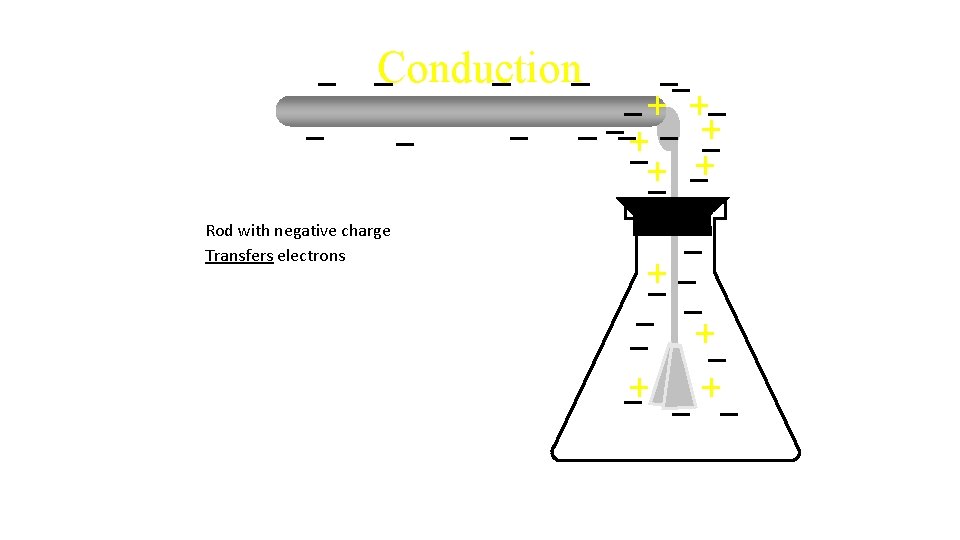
Conduction Rod with negative charge

Conduction Rod with negative charge

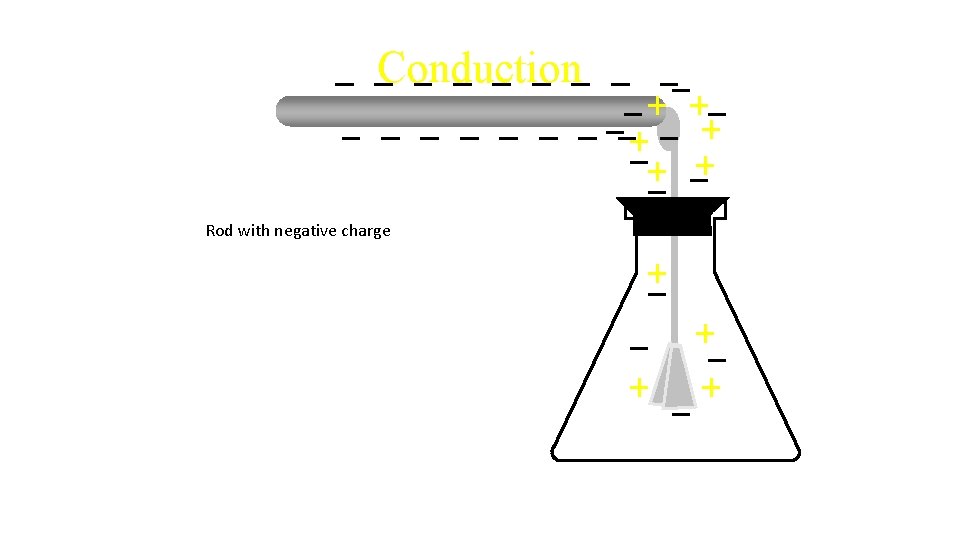
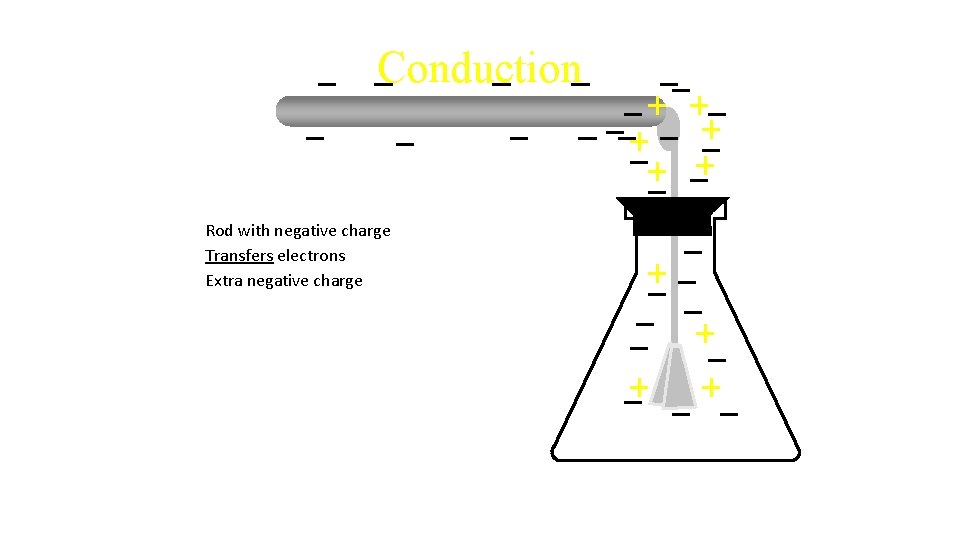
Conduction Rod with negative charge Transfers electrons

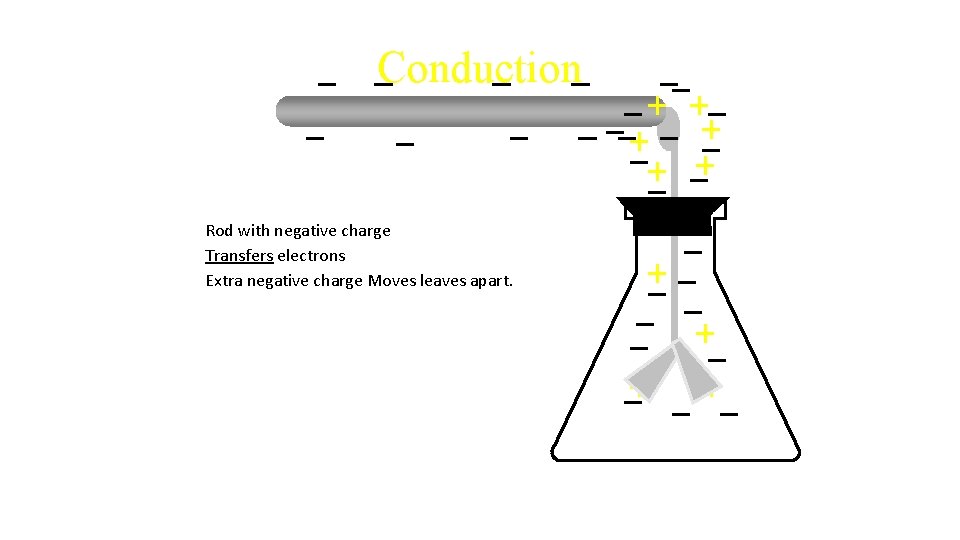
Conduction Rod with negative charge Transfers electrons Extra negative charge

Conduction Rod with negative charge Transfers electrons Extra negative charge Moves leaves apart.

Since. Consider static electricity stationary charge put) whichisballoon you can (stays charge byonly some canand maintain charge rubbing onmaterials your hair have astick to a wall Insulators: Electrical charge is localized - fixed Electrons can’t move around ____ __ Rubber, plastic, cloth, glass, wood Conductors: Electrical charge spreads through material Electrons move around, weakening charge Most metals are good conductors _ _ _

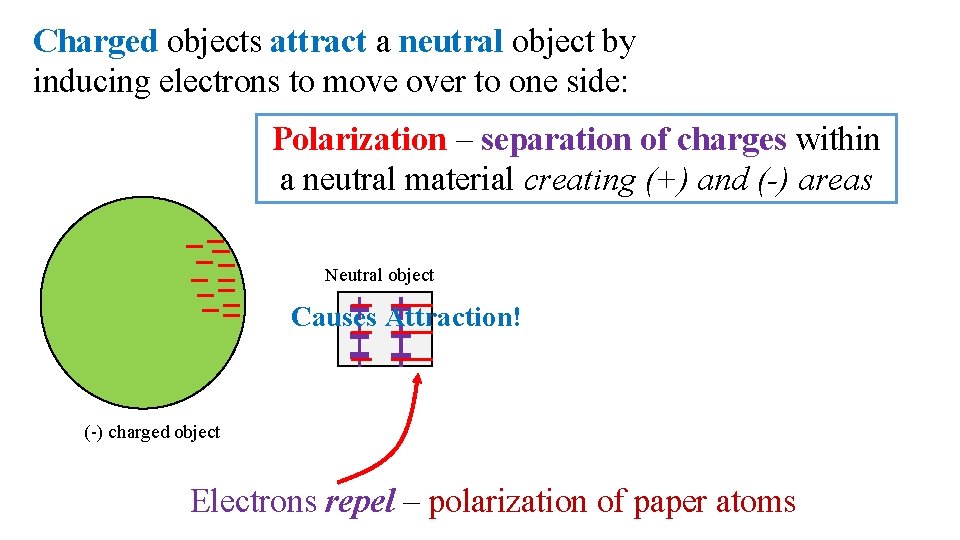
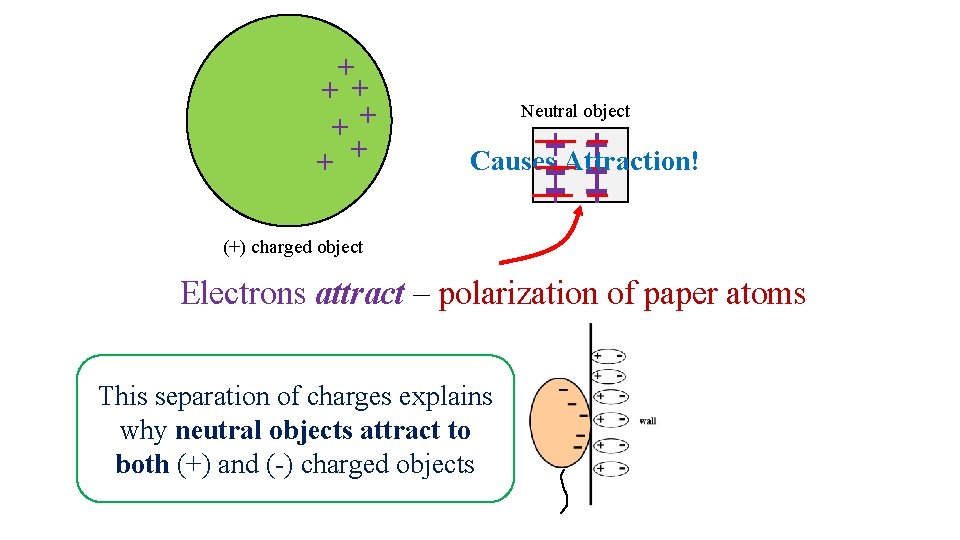
Charged objects attract a neutral object by inducing electrons to move over to one side: Polarization – separation of charges within a neutral material creating (+) and (-) areas Neutral object Causes Attraction! (-) charged object Electrons repel – polarization of paper atoms

+ + + + Neutral object Causes Attraction! (+) charged object Electrons attract – polarization of paper atoms This separation of charges explains why neutral objects attract to both (+) and (-) charged objects

Static discharge • Eventually static electric charge will move. • Slowly the electrons may move into moisture in the air • Or quickly in a spark.

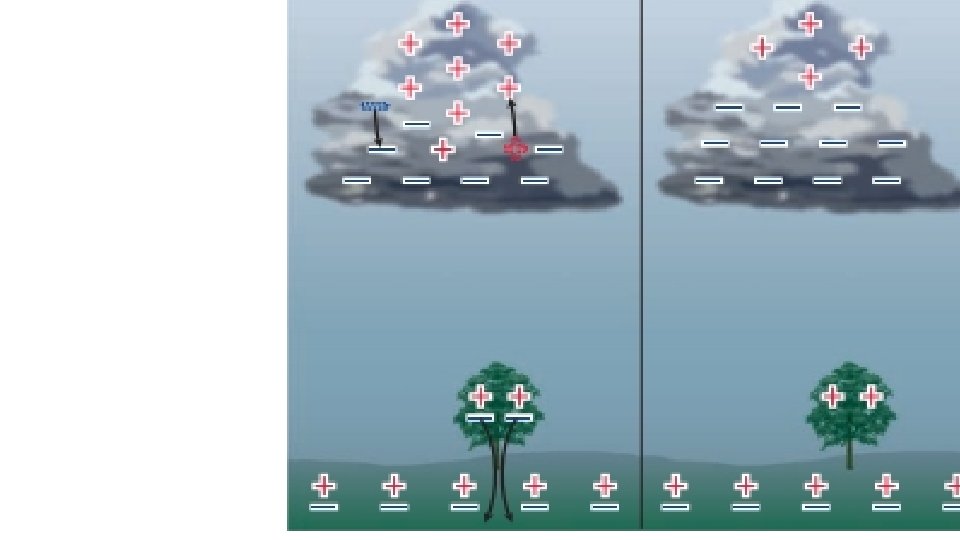
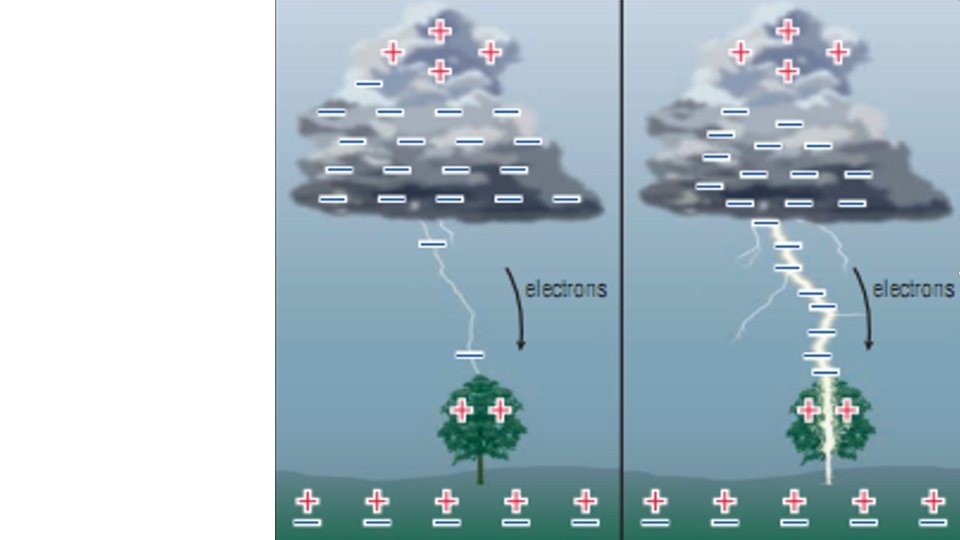
What Causes Lightning? • Lightning is actually just static electricity on a much larger scale. • The rubbing is caused by air moving around • In thunderclouds bottom is usually negative and top is positive.



Lightning is (-) charge in the clouds causing polarization in the surface of the Earth followed by a shock of electrons between the two

CAN YOU ANSWER THESE QUESTIONS? S 1 -3 -04: How does the Atomic Model help to explain static electricity? Vocabulary & Concepts Neutral Conservation of Charge Insulator Conductor Polarization