States of Matter What are three states of























- Slides: 35

States of Matter

What are three states of matter?

What are three states of matter? Solid, liquid, & gas

What are three states of matter? Solid, liquid, & gas What is the 4 th state of matter?

What are three states of matter? Solid, liquid, & gas What is the 4 th state of matter? Plasma

The three states of matter are solid, liquid & gas. Think about water, it comes in three different forms. Ice - solid water - liquid vapour - gas

Terms: Volume - the amount of space occupied by an object Compressed - pushed together; squeezed.

How can we tell the three states apart?

Gas A gas takes on the volume and shape of any container it is put into; a gas can flow. Also the volume of a gas can change. You have to cap a container that has a gas in it or it will escape. You can compress a gas. For example when you put air in a bicycle tire you squeeze it in or compress it.

Liquid A liquid has a set volume but it will have the shape of the container it is in. A liquid flows to take the shape of its container but it does not change in volume. For example the amount of juice stays the same whether it is in the box, a juice container or a bowl.

Solid A solid has a set volume and shape; it cannot flow. A solid does not change its shape and it cannot be compressed. For example a block of wood or a sheet of steel does not flow and it cannot be compressed.

Gas - a state matter that has no definite shape or volume. Liquid - a state of matter that has a definite volume but no definite shape Solid - a state of matter that has a definite volume and shape


The Particle Theory

There are five main points of the particle model of matter that we will be looking at:

1. All substances are made of tiny particles.

2. The particles are always in motion.

3. The particles have spaces between them.

4. Particles are always in motion. The motion of the particles increases/decreases when the temperature increase/decreases.

5. There attractive forces between the particles.

The particle model relates to the states of matter.

SOLIDS In a solid, the particles are closely packed, held by strong attractive forces, but able to vibrate and rotate in position.

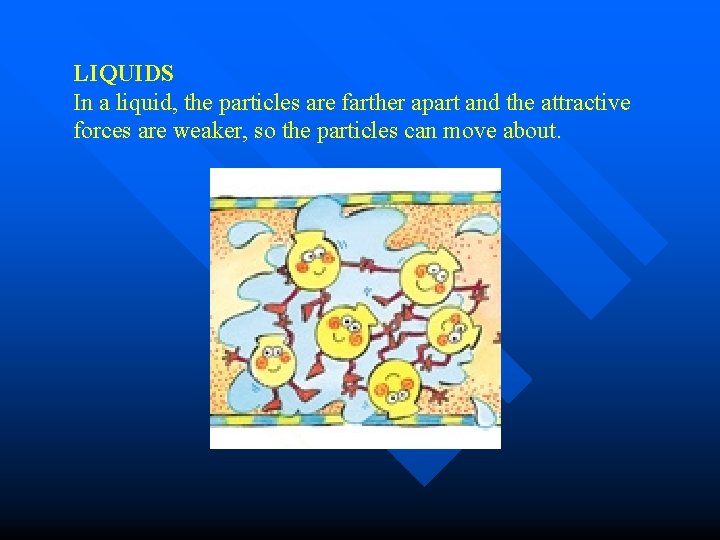
LIQUIDS In a liquid, the particles are farther apart and the attractive forces are weaker, so the particles can move about.

Thermal Energy Thermal energy is the energy generated by the movement or vibration of particles; the total kinetic energy of all the particles in a substance. As the motion of the particles in a substance change the temperature of the substance changes.

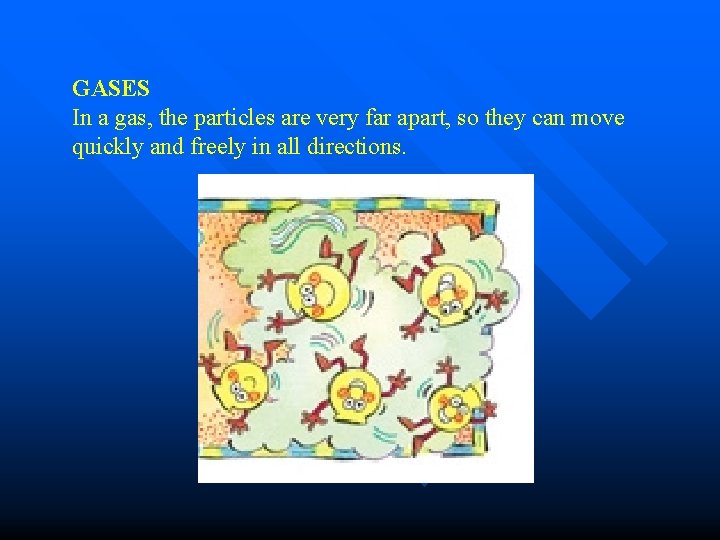
GASES In a gas, the particles are very far apart, so they can move quickly and freely in all directions.

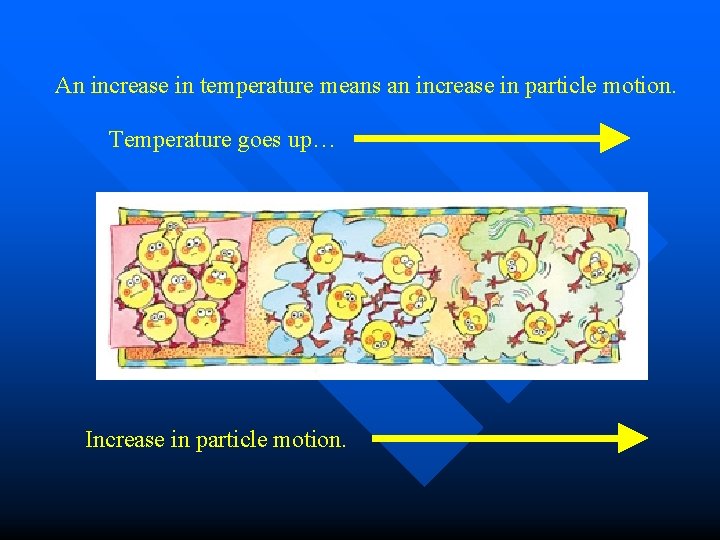
An increase in temperature means an increase in particle motion. Temperature goes up… Increase in particle motion.

Temperature indicates the average speed of particle motion in a substance.

How the Particle Model Explains Changes in State

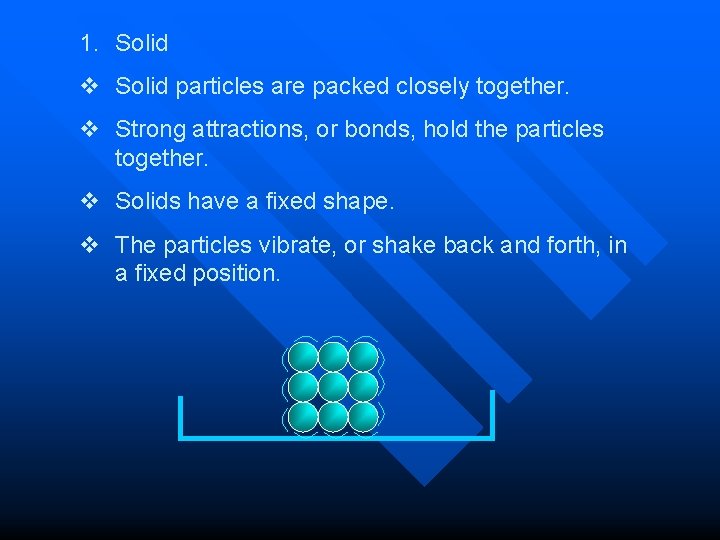
1. Solid v Solid particles are packed closely together. v Strong attractions, or bonds, hold the particles together. v Solids have a fixed shape. v The particles vibrate, or shake back and forth, in a fixed position.

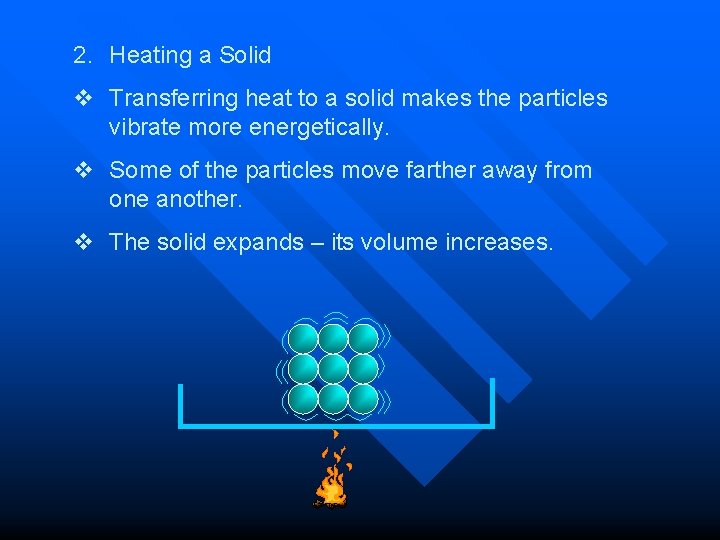
2. Heating a Solid v Transferring heat to a solid makes the particles vibrate more energetically. v Some of the particles move farther away from one another. v The solid expands – its volume increases.

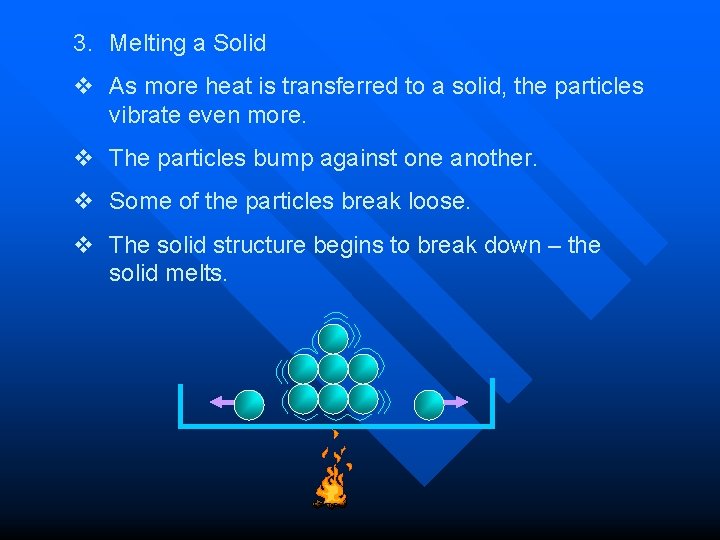
3. Melting a Solid v As more heat is transferred to a solid, the particles vibrate even more. v The particles bump against one another. v Some of the particles break loose. v The solid structure begins to break down – the solid melts.

4. Liquid v The particles have more kinetic energy to move about. v The bonds that hold the particles together are weak. v Liquids take on the shape of their containers.

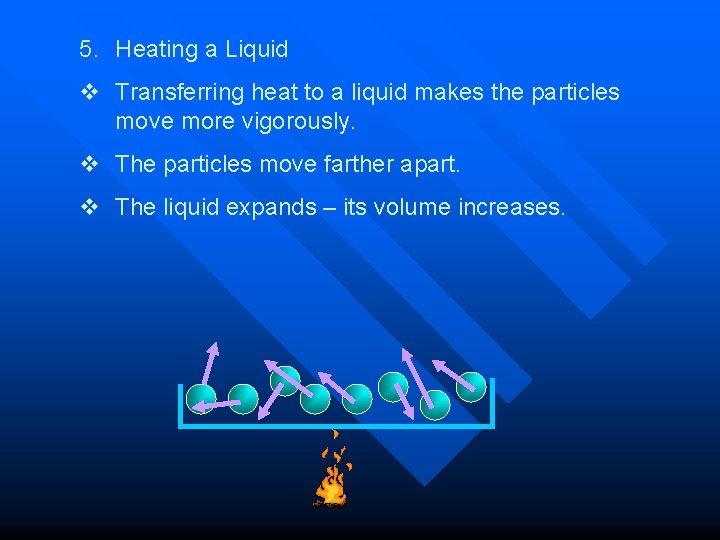
5. Heating a Liquid v Transferring heat to a liquid makes the particles move more vigorously. v The particles move farther apart. v The liquid expands – its volume increases.

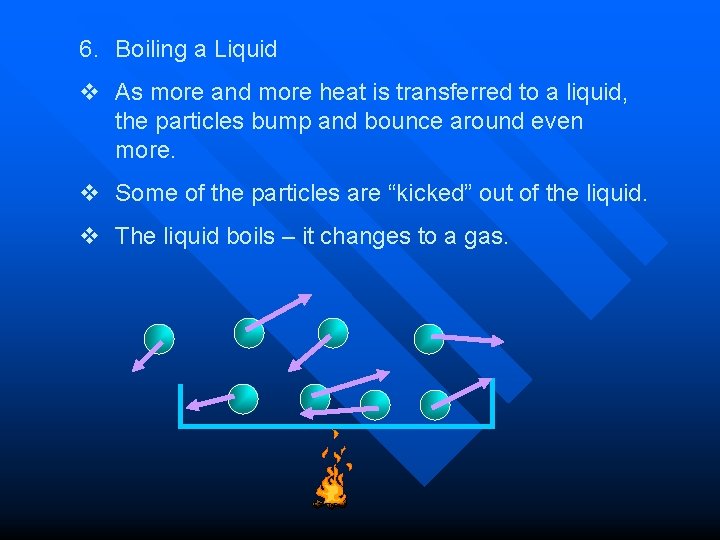
6. Boiling a Liquid v As more and more heat is transferred to a liquid, the particles bump and bounce around even more. v Some of the particles are “kicked” out of the liquid. v The liquid boils – it changes to a gas.

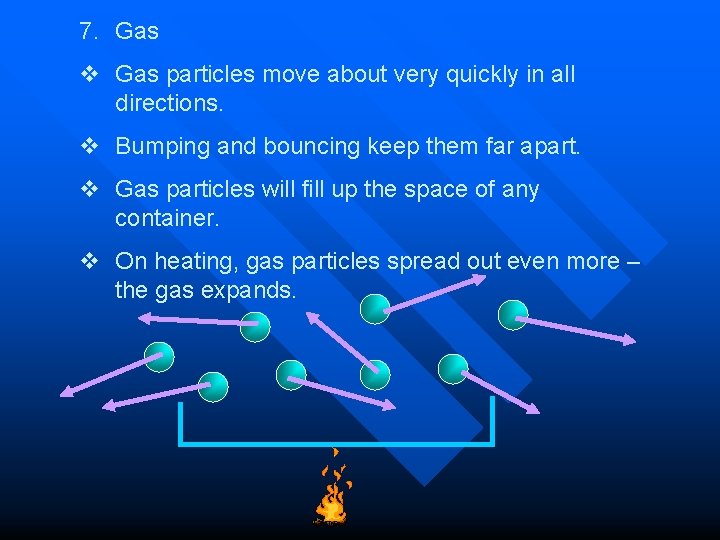
7. Gas v Gas particles move about very quickly in all directions. v Bumping and bouncing keep them far apart. v Gas particles will fill up the space of any container. v On heating, gas particles spread out even more – the gas expands.