States of Matter The Kinetic Molecular Theory KMT






- Slides: 17

States of Matter

The Kinetic Molecular Theory • KMT – describes the behavior of gases in terms of particles in motion Size: • Gas particles are small • Spaced very far apart • No significant attractive or repulsive forces between the gas particles

The Kinetic Molecular Theory Motion: • Gas particles are in constant random motion • Kinetic energy is transferred when particles collide with each other or the sides of their container • The collisions are elastic (no energy is lost)

The Kinetic Molecular Theory Energy: • Depends on the particles mass and velocity • KE = ½ mv 2 • Temperature is a measure of the average kinetic energy of the particles in a gas • 2 gases at the same temperature have the same average kinetic energy

Explaining the behavior of gases Compression and expansion: • A gas will expand to fill its container • Random motion of gas particles fill the empty spaces and it expands until the container stops it. • Large amounts of empty space between gas particles allow it to be compressed (squeezed into a smaller space)

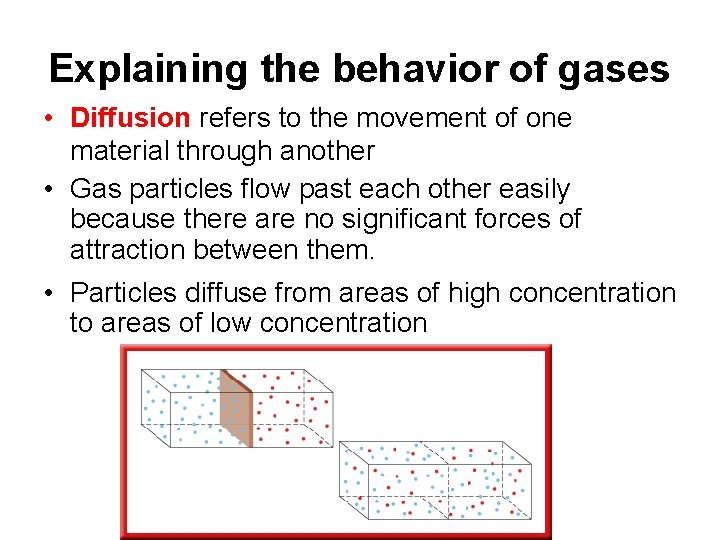
Explaining the behavior of gases • Diffusion refers to the movement of one material through another • Gas particles flow past each other easily because there are no significant forces of attraction between them. • Particles diffuse from areas of high concentration to areas of low concentration

Explaining the behavior of gases Effusion – a gas escaping through a tiny opening • Gases with a higher mass effuse slower than gases with less mass Example: A tire deflating from a puncture

Gas pressure Pressure is force per unit area. • When gas particles collide with the walls of their container, they exert pressure on the walls. • SI unit for pressure is the Pascal (Pa) • 1 atm = 101. 3 k. Pa = 760 mm Hg = 760 Torr – Barometer = instrument that measures pressure of the atmosphere – Manometer = instrument that measures pressure of a gas in a closed container

Temperature • Applies to solids, liquids, and gases • Increasing the temp causes an increase in the average kinetic energy of the particles – At a given temp, all particles of a substance have the same average kinetic energy • Kelvin (o. C+273) = SI unit of temp • Absolute zero = lowest temp on Kelvin scale (0 K = -273 o. C ~ -460 o. F) – Movement of all particles stops at this temp

Forces of Attraction • The attractive forces that hold particles together in ionic and covalent bonds are called intramolecular forces. (within the molecule) • Intermolecular forces hold particles of different molecules together. (between molecules) • There are three types of intermolecular forces: dispersion forces, dipole–dipole forces, and hydrogen bonds.

Liquids: • Fixed volume, no fixed shape • Densities are much greater than that of gases • Particles are packed closer together • Is a fluid – has the ability to flow and change shape

Liquids Viscosity – measure of the resistance of a liquid to flow • Example: water vs. molasses • Viscosity is affected by the type of intermolecular forces and by the temperature • As temperature increases, viscosity decreases

Liquids Surface Tension: The particles on the surface of a liquid are being pulled down by intermolecular forces (between two molecules) • The stronger the attraction between particles the stronger the surface tension

Liquids Capillary action occurs when adhesive forces are greater than cohesive forces • Adhesion is the force of attraction between molecules that are different, such as water molecules and the molecules of silicon dioxide in glass. • Cohesion is the force of attraction between identical molecules, such as water molecules Example: water in a graduated cylinder

Solids: • Particles in a solid are in constant motion (vibrating) • Definite shape and volume • Very strong attractive forces acting between molecules • More dense than most liquids • Most solids are in the shape of crystals (repeating geometric patterns)

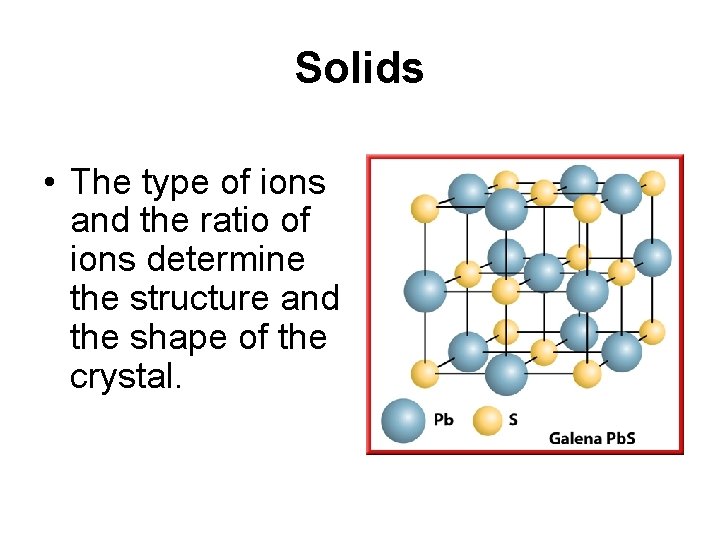
Solids • The type of ions and the ratio of ions determine the structure and the shape of the crystal.

Solids • The particles in an amorphous solid are not arranged in a regular, repeating pattern and do not form crystals (they can take on different shapes). • Examples of amorphous solids include glass, rubber, and many plastics.