States of Matter Solids Liquids Gases Intermolecular Forces













- Slides: 26

States of Matter Solids Liquids Gases

Intermolecular Forces Intermolecular forces are attractions that bring particles together. Not exactly a Bond… just an attraction.

Intermolecular Forces Three Kinds… 1. Dispersion Forces 2. Dipole-Dipole Forces 3. Hydrogen Bonds

Dispersion Forces Weak forces that come from electrons when they are unbalanced.

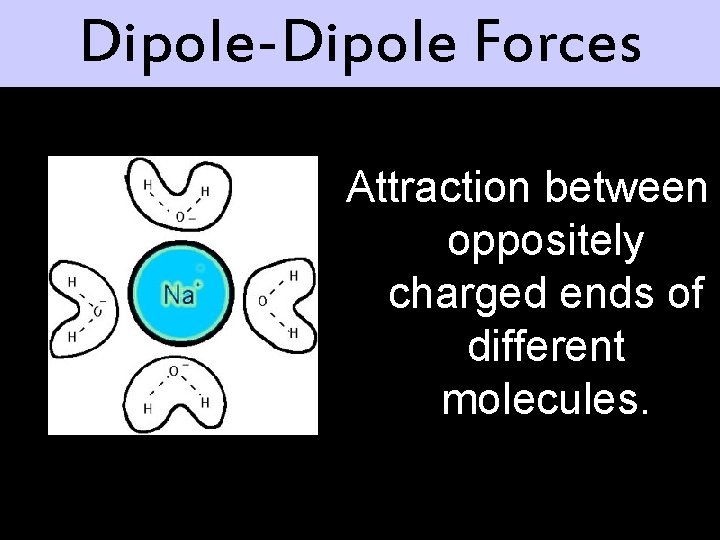
Dipole-Dipole Forces Attraction between oppositely charged ends of different molecules.

Hydrogen Bonds (a form of dipole-dipole bond) A hydrogen atom on one molecule being attracted to a lone pair of electrons on another atom.

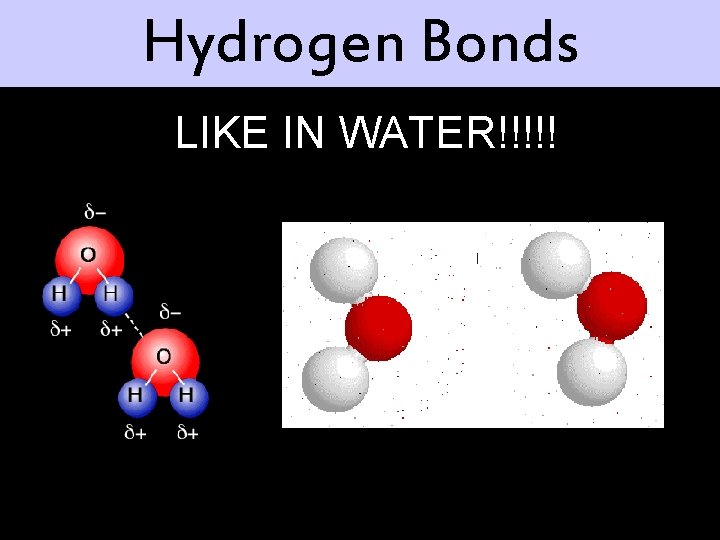
Hydrogen Bonds LIKE IN WATER!!!!!

Liquids and Solids Density and Compression Liquid vs. Gas Fluidity Diffusion of color through water (rate depends on water temp)

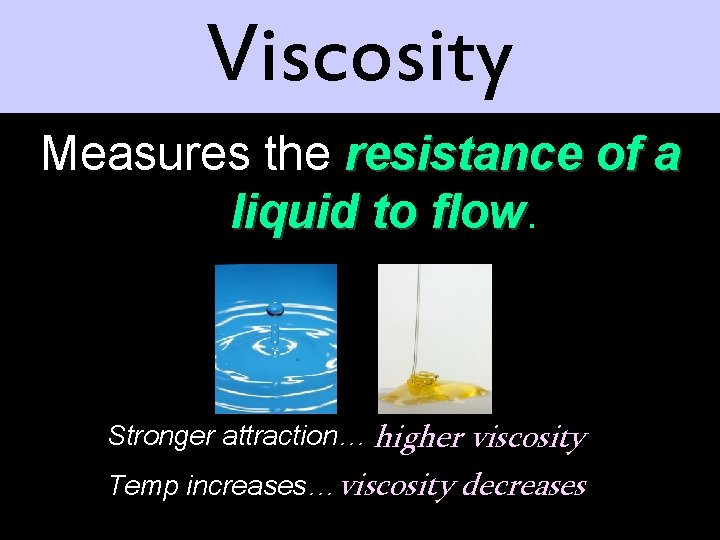
Viscosity Measures the resistance of a liquid to flow Stronger attraction… higher viscosity Temp increases… viscosity decreases

Surface Tension Energy needed to increase the surface area of a liquid. Surfactants decrease surface tension (get in the way of bonds)


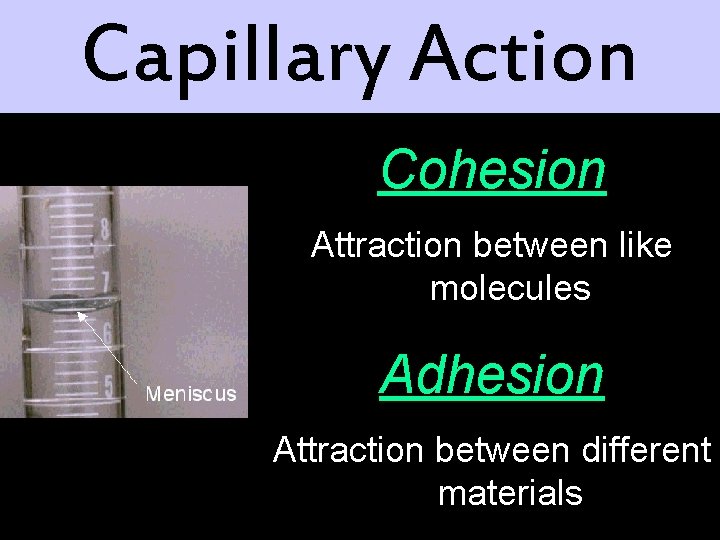
Capillary Action Cohesion Attraction between like molecules Adhesion Attraction between different materials

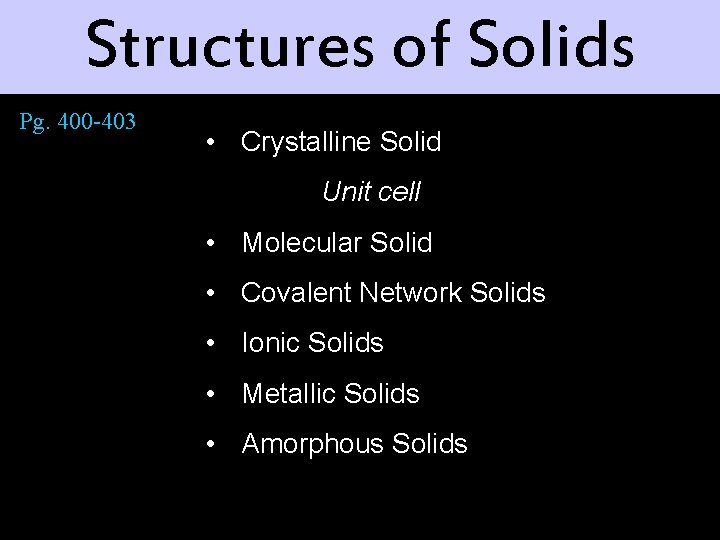
Structures of Solids Pg. 400 -403 • Crystalline Solid Unit cell • Molecular Solid • Covalent Network Solids • Ionic Solids • Metallic Solids • Amorphous Solids

Phase Changes

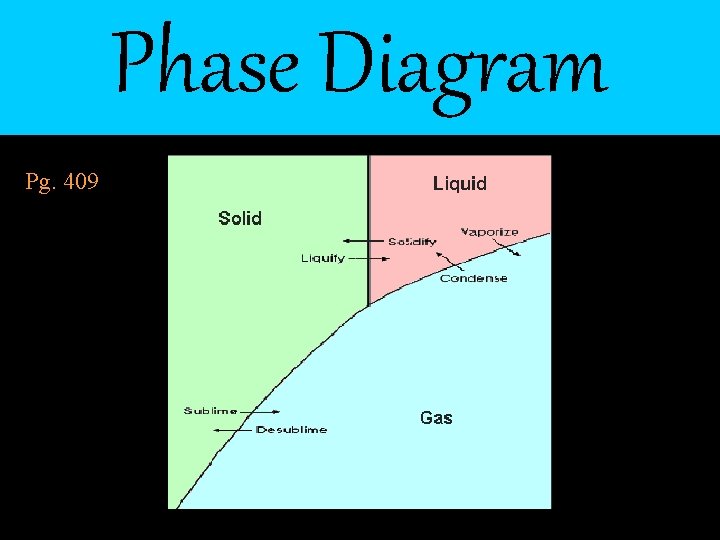
Phase Diagram Pg. 409

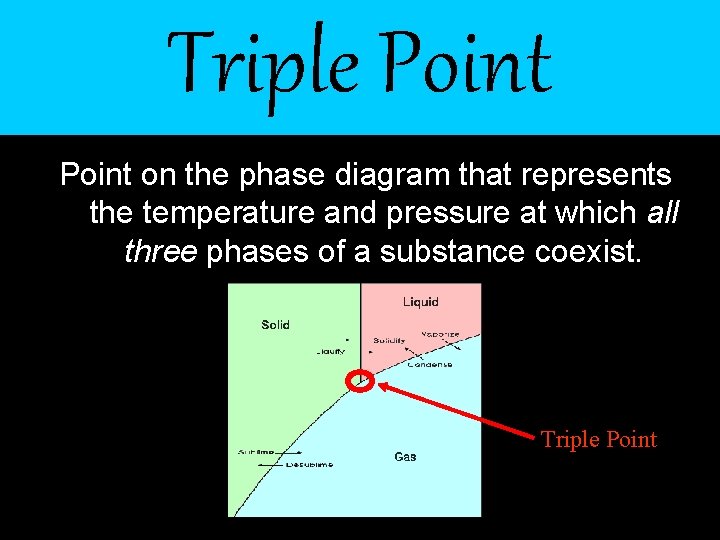
Triple Point on the phase diagram that represents the temperature and pressure at which all three phases of a substance coexist. Triple Point

MELTING Solid Liquid Melting Point: temperature at which forces holding a solid together are broken and it becomes a liquid.

Vaporization Liquid Gas Evaporation: vaporization that occurs at the surface of a liquid.

Is evaporation a heating or cooling process?

Vapor Pressure exerted by a vapor over a liquid.

Boiling Point Temp at which… vapor pressure = atmospheric pressure for a liquid.

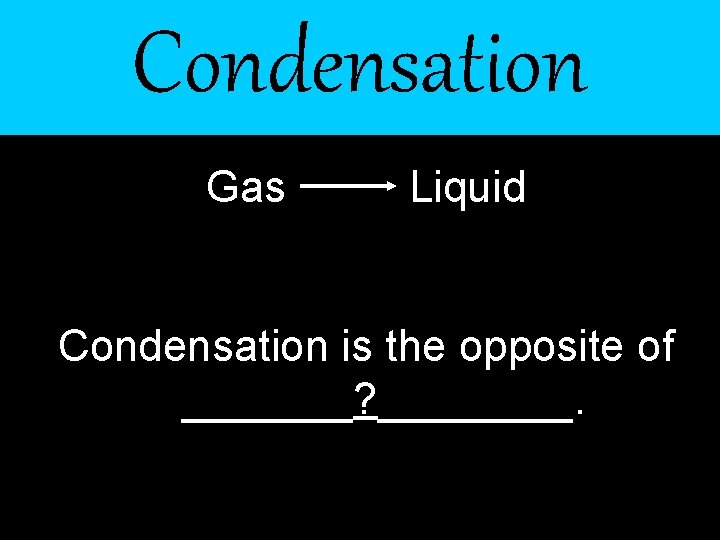
Condensation Gas Liquid Condensation is the opposite of _______? ____.

Freezing Point Liquid Solid Temp at which a liquid is converted into a solid.

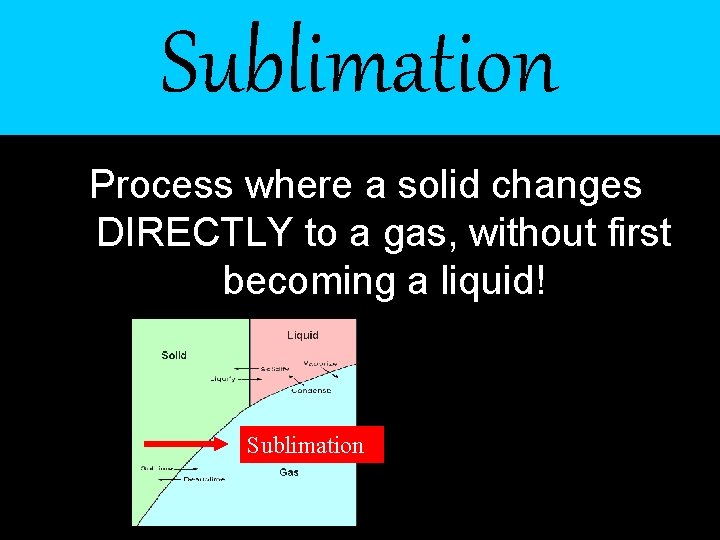
Sublimation Process where a solid changes DIRECTLY to a gas, without first becoming a liquid! Sublimation

Freeze Dried Foods

Deposition Gas Solid When a substance changes from a vapor/gas to a solid, without first becoming a liquid.