States of Matter Solids liquids and gases Why









- Slides: 14

States of Matter Solids, liquids, and gases

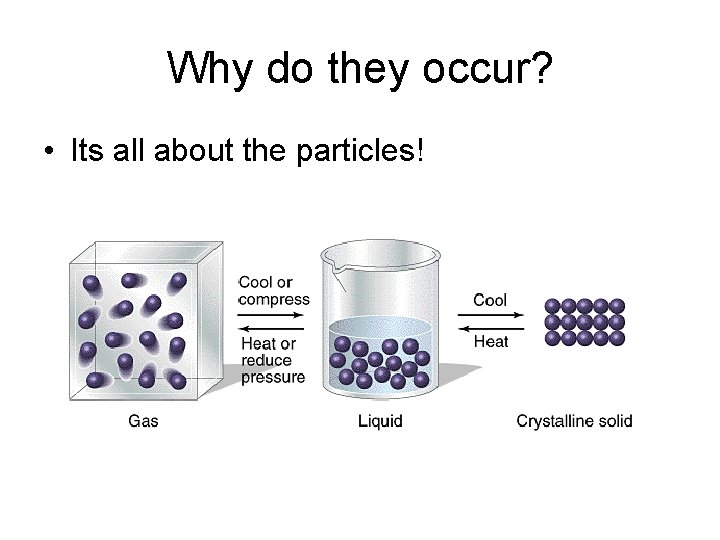
Why do they occur? • Its all about the particles!

Solids • Molecules are packed tightly together • Little free space between particles • Particles do not move around much

Liquid • Particles are close together (but not as close as they are in a solid) • Particles move faster than in a solid • Particles can move freely past one another

Gas • Particles are spread out with lots of free space in between particles • Particles are moving faster than in a liquid • Particles can move freely past one another

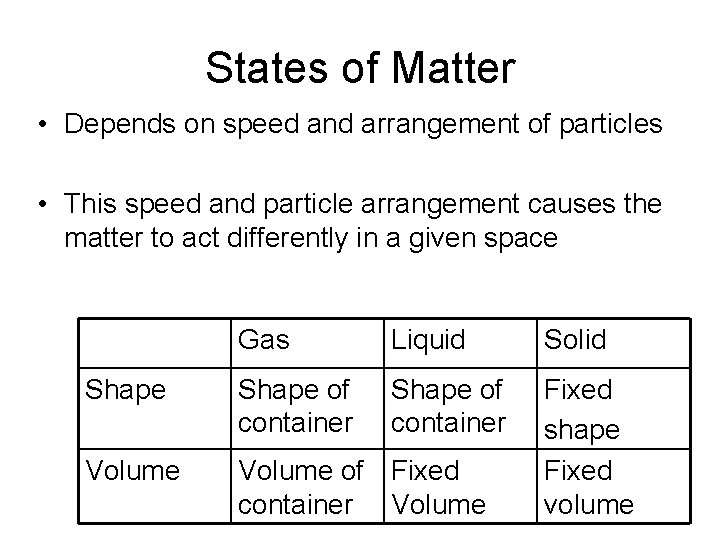
States of Matter • Depends on speed and arrangement of particles • This speed and particle arrangement causes the matter to act differently in a given space Gas Liquid Solid Shape of container Volume of Fixed container Volume Fixed shape Fixed volume

States of Matter • All matter is constantly moving • The only way to stop all particle movement is to reach absolute zero • − 459. 67°

Changing the State of Matter Vaporization, freezing, melting, condensation, sublimation

What causes a change in state? • Adding or taking away energy (i. e. heat) • Endothermic: adding heat to a substance. Forces holding particles together are weakened (Endothermic means heat goes “in”) • Exothermic: taking heat away or cooling a substance. Forces holding particles together are strengthened (Exothermic means heat “exits”)

Vaporization • Liquid Gas • Do you think this would be endothermic or exothermic? • Answer: Endothermic • Example: Steam

Freezing • Liquid Solid • Do you think this would be endothermic or exothermic? • Answer: Exothermic • Example: Making ice cubes

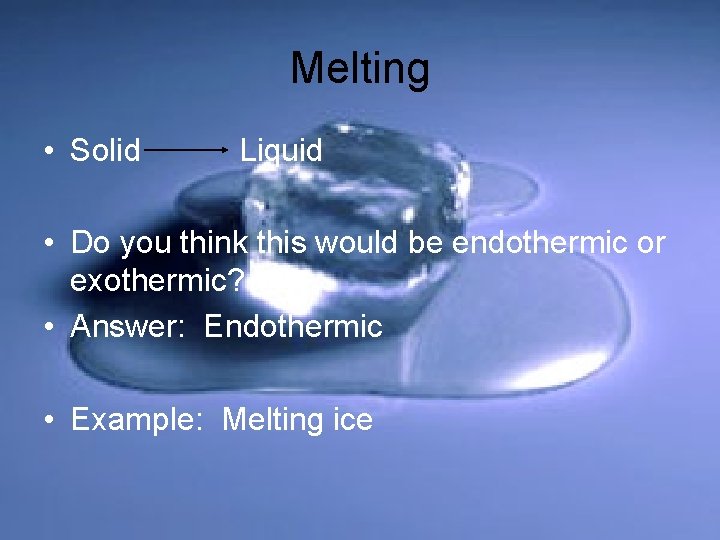
Melting • Solid Liquid • Do you think this would be endothermic or exothermic? • Answer: Endothermic • Example: Melting ice

Condensation • Gas Liquid • Do you think this would be endothermic or exothermic? • Answer: Exothermic • Example: formation of rain from water vapor; dew on the grass, condensation on windows

Sublimation • Solid gas • Do you think this would be endothermic or exothermic? • Answer: Endothermic • Example: Dry ice (frozen CO 2)