States of Matter Phase Changes Heat of Fusion







- Slides: 13

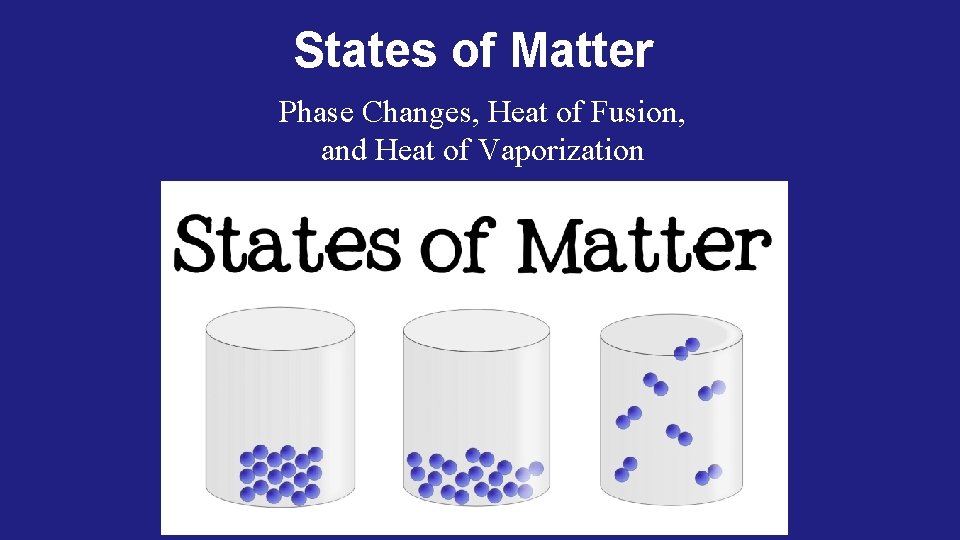
States of Matter Phase Changes, Heat of Fusion, and Heat of Vaporization

Phases of Matter: (review) Solid matter that has definite volume and definite shape Liquid matter that has definite volume but indefinite shape Gas matter that has indefinite volume and indefinite shape

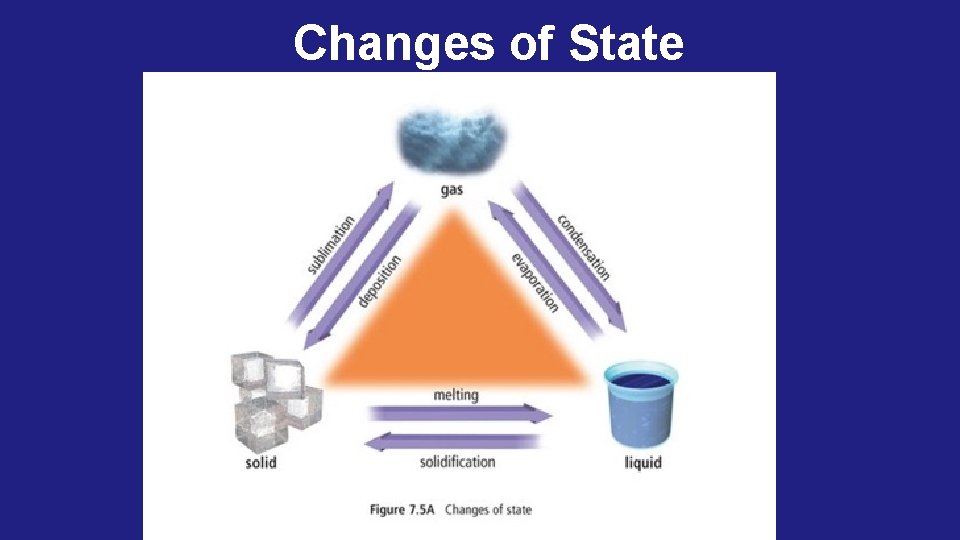
Phase Change Descriptions: Melting the change from solid to liquid. Freezing the change from liquid to solid. Evaporation (vaporization) the change from liquid to gas. Condensation the change from gas to liquid. Sublimation the change from solid to gas. Deposition the change from gas to solid.

Changes of State

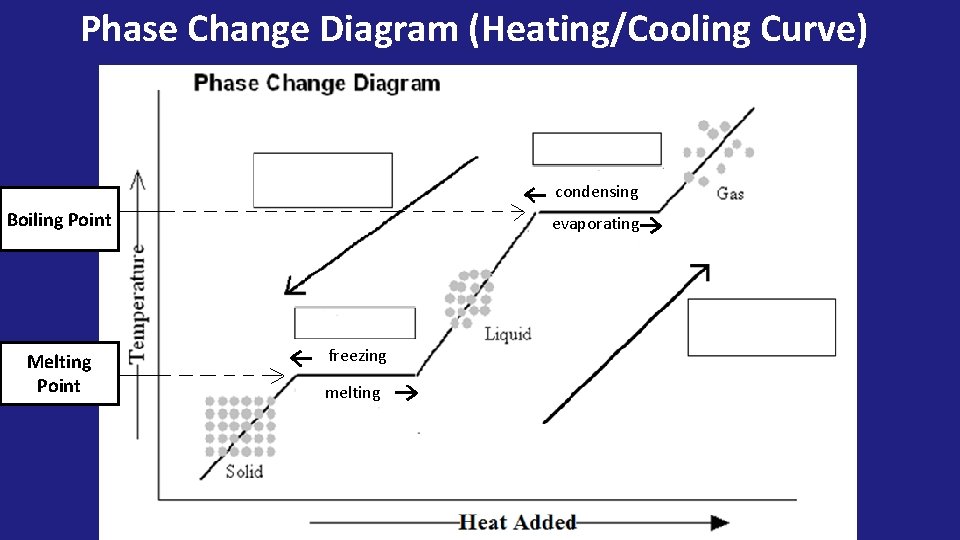
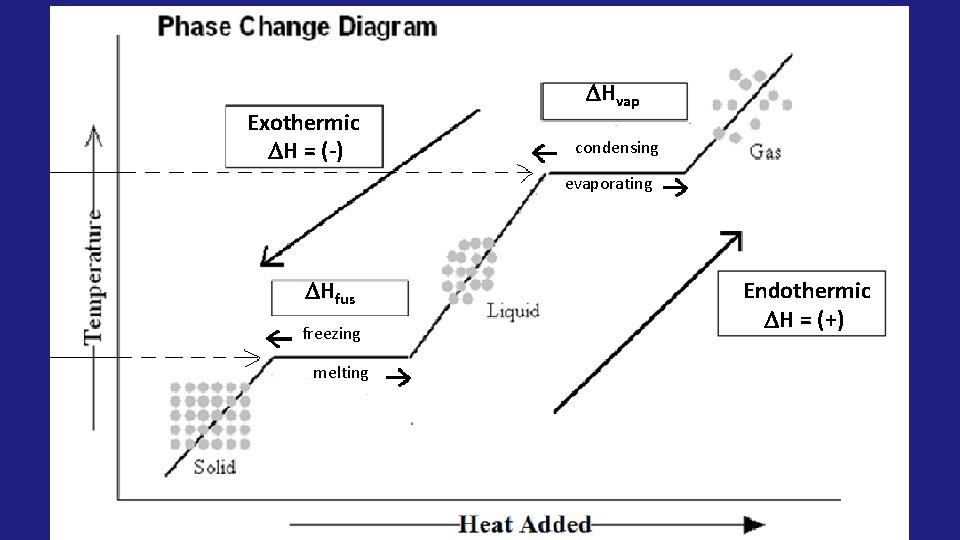
Phase Change Diagram (Heating/Cooling Curve) condensing Boiling Point Melting Point evaporating freezing melting

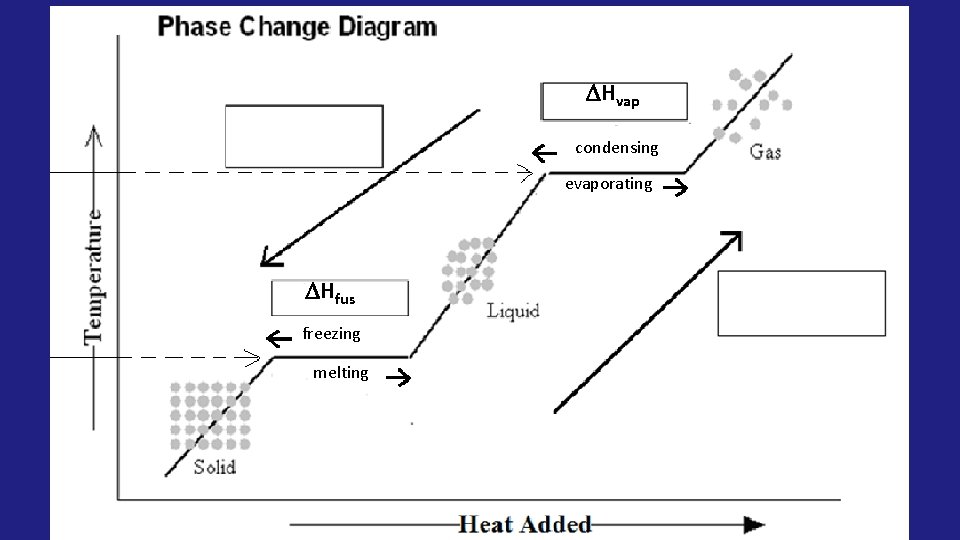
• Heat of fusion: ( Hfus) the energyabsorbed to change one mole of a substance from a solid to a liquid. (melting) Heat of vaporization: ( Hvap) the energy absorbed to change one mole of a substance from liquid to •

• NO TEMPERATURE CHANGE OCCURS DURING A PHASE CHANGE!

Hvap condensing evaporating Hfus freezing melting

• When moving UP the curve, the Hfus and Hvap will be POSITIVE (+) because they are endothermic processes • When moving DOWN the curve, the Hfus and Hvap will be NEGATIVE (-) because they are exothermic processes

Exothermic H = (-) Hvap condensing evaporating Hfus freezing melting Endothermic H = (+)

Calculations with Phase Changes Example 1: How much energy would it take to completely melt a 7. 20 mol sample of ice at 0°C? The heat of fusion ( Hfus) of H 2 O is 6. 02 k. J/mol.

• Calculations with Phase Changes Example 2: How much energy would it take to completely melt a 15. 0 g sample of ice at 0°C? The heat of fusion ( Hfus) of H 2 O is 6. 02 k. J/mol. What must we do first?

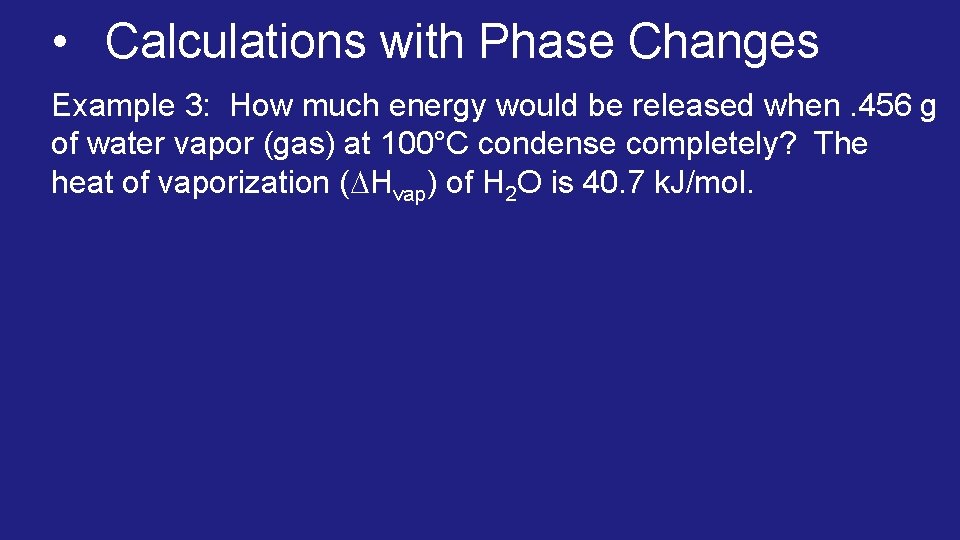
• Calculations with Phase Changes Example 3: How much energy would be released when. 456 g of water vapor (gas) at 100°C condense completely? The heat of vaporization ( Hvap) of H 2 O is 40. 7 k. J/mol.