STATES OF MATTER Kinetic Theory of Matter is
















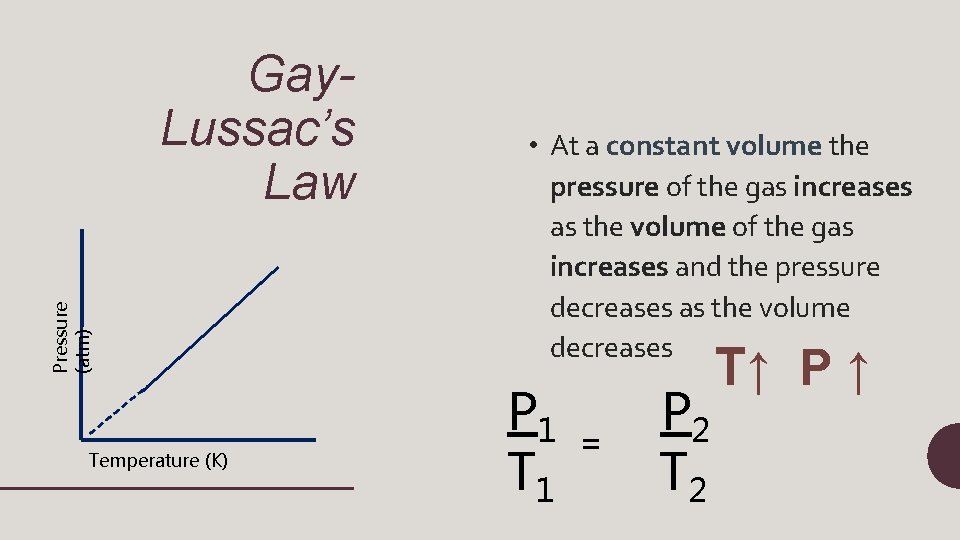





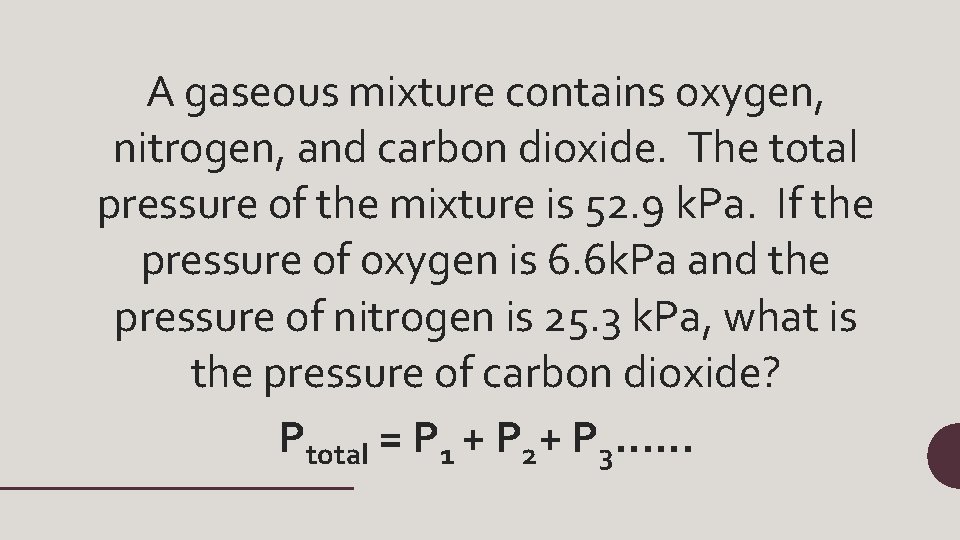
- Slides: 59

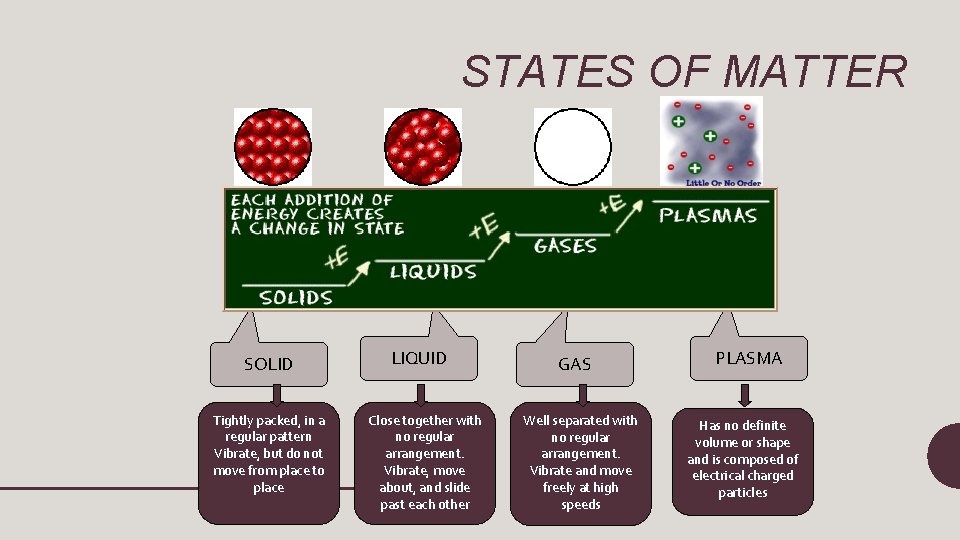
STATES OF MATTER

Kinetic Theory of Matter is made up of particles which are in continual random motion.

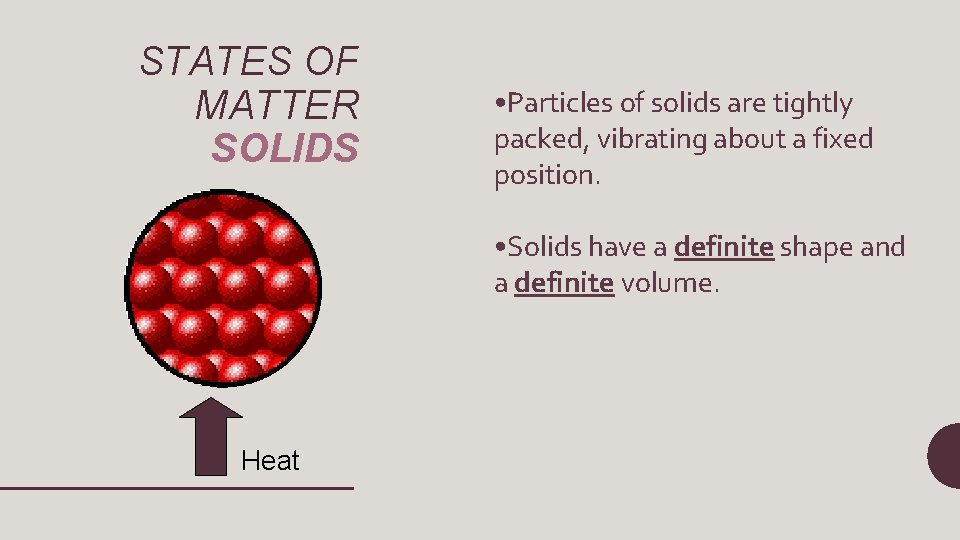
STATES OF MATTER SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. • Solids have a definite shape and a definite volume. Heat

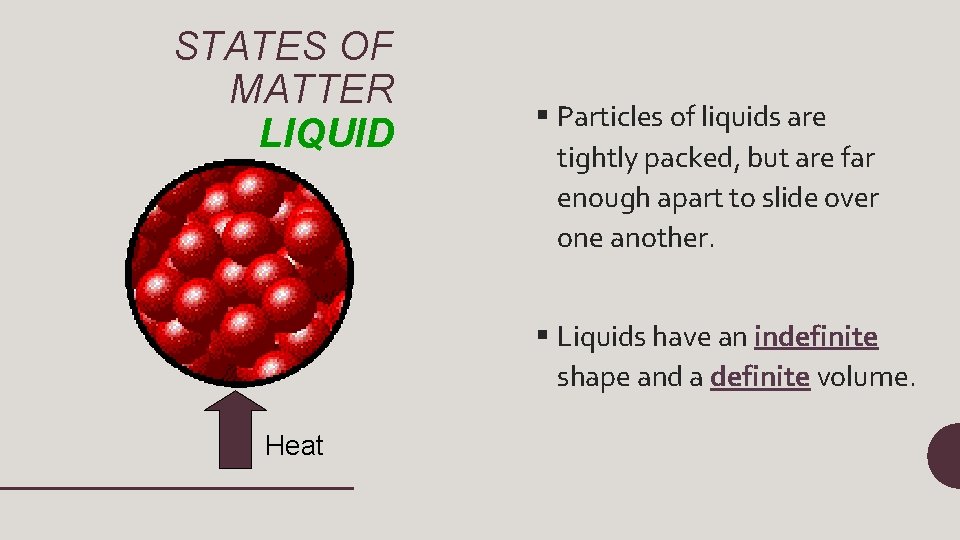
STATES OF MATTER LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume. Heat

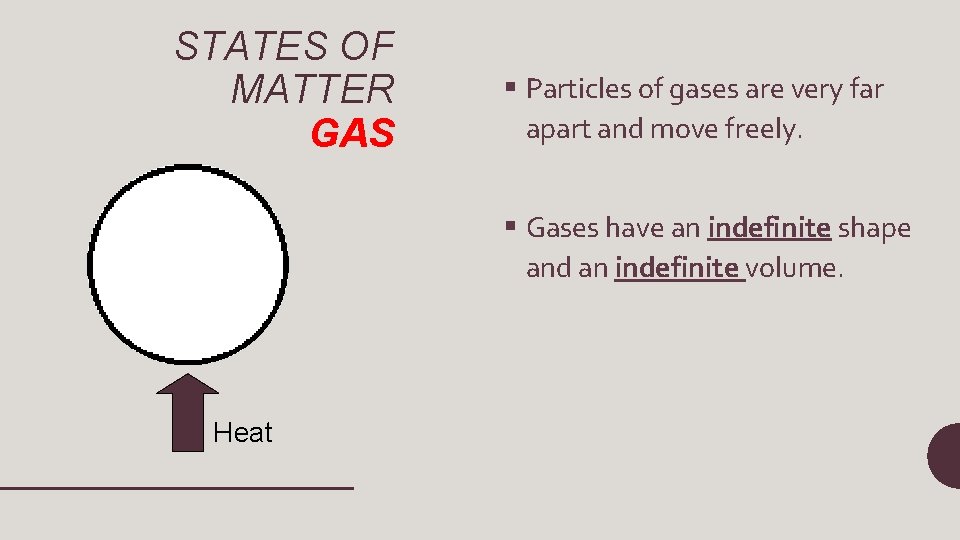
STATES OF MATTER GAS § Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume. Heat

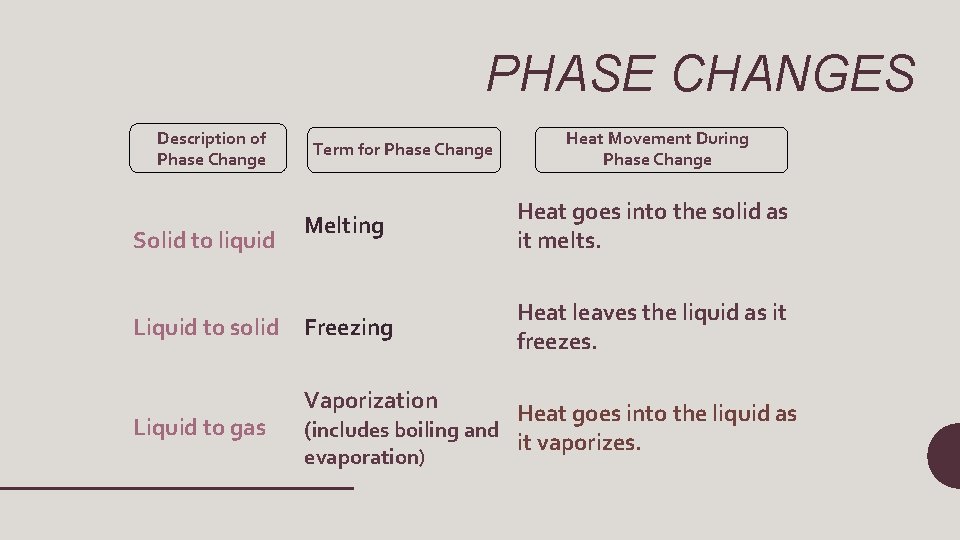
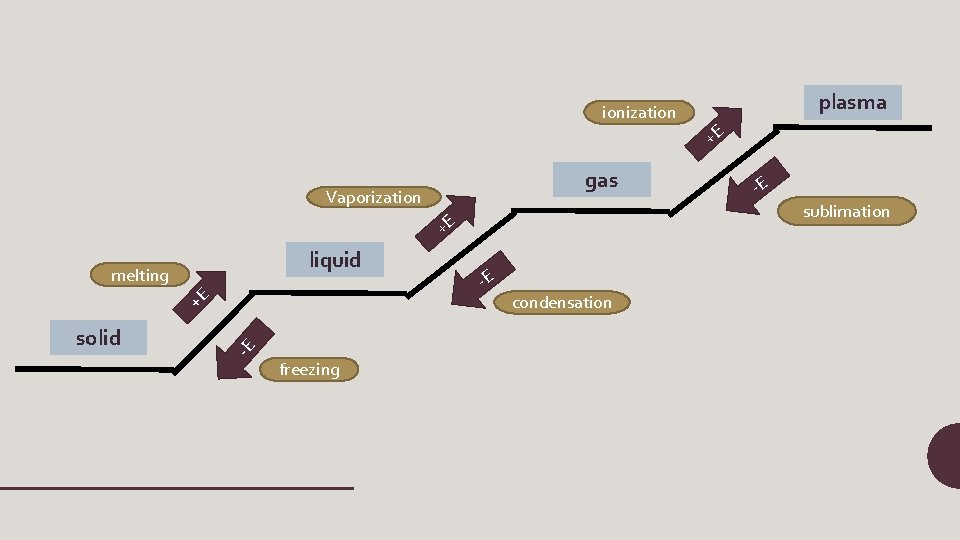
PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Melting Heat goes into the solid as it melts. Liquid to solid Freezing Heat leaves the liquid as it freezes. Liquid to gas Vaporization Heat goes into the liquid as (includes boiling and it vaporizes. Solid to liquid evaporation)

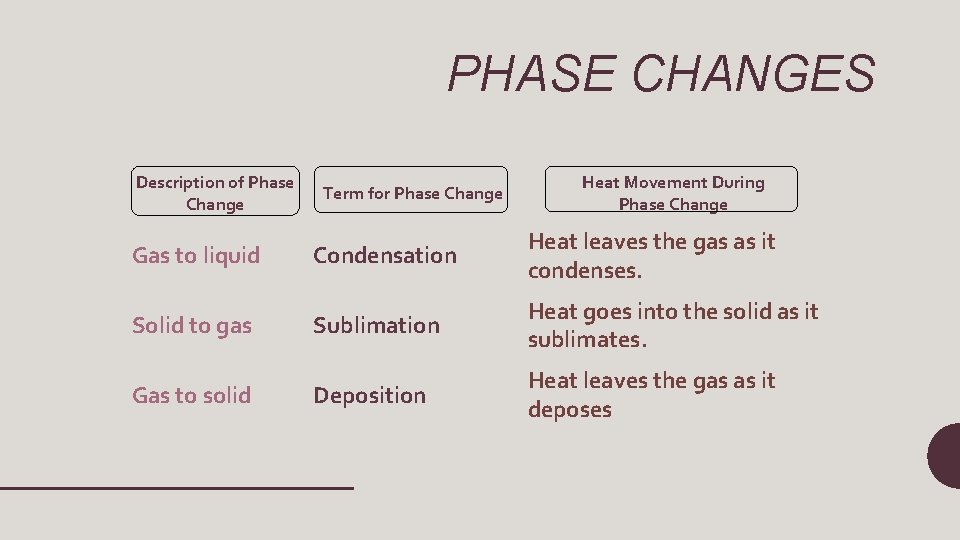
PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Condensation Heat leaves the gas as it condenses. Solid to gas Sublimation Heat goes into the solid as it sublimates. Gas to solid Deposition Heat leaves the gas as it deposes Gas to liquid

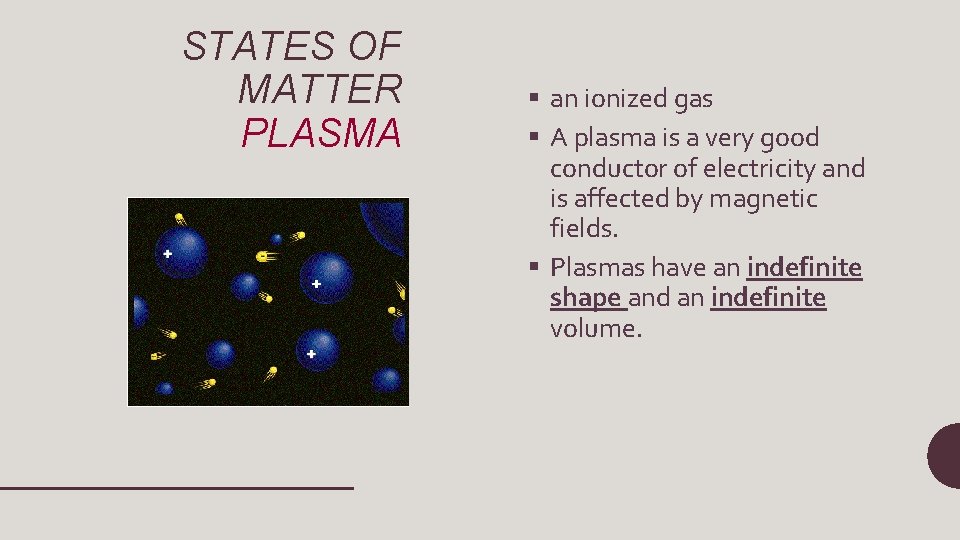
STATES OF MATTER PLASMA § an ionized gas § A plasma is a very good conductor of electricity and is affected by magnetic fields. § Plasmas have an indefinite shape and an indefinite volume.


STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

ionization gas Vaporization solid -E +E melting freezing +E -E sublimation +E liquid plasma -E condensation

Is evaporation endothermic or exothermic? 1. Fastest molecules leave from the liquid to the gas phase 2. Now there is a decrease in Ave. KE 3. To equilibrate - heat will flow into the system (thus endothermic)

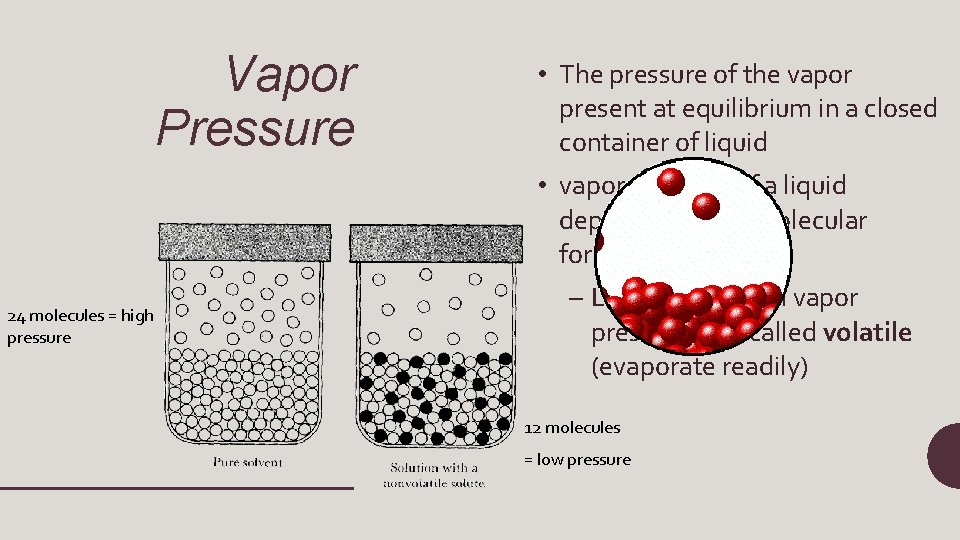
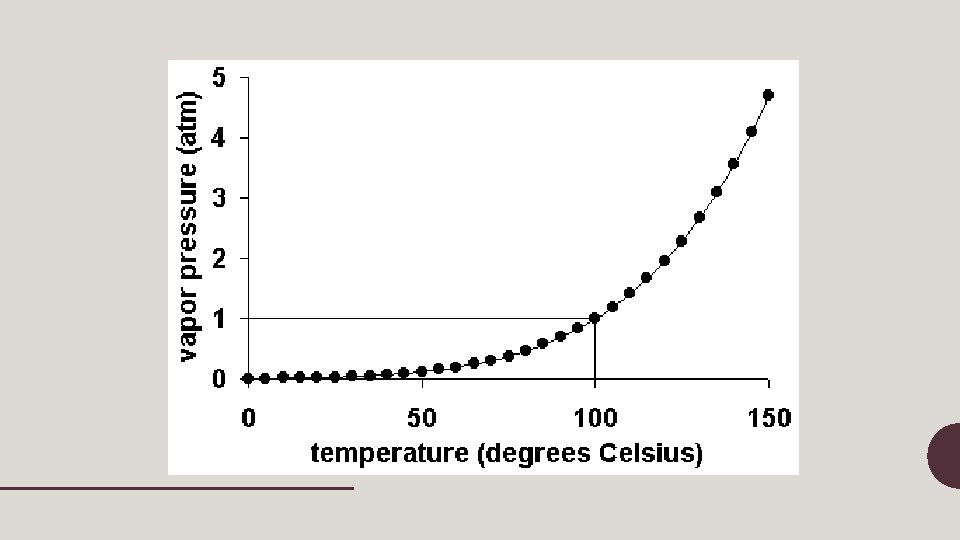
Vapor Pressure • The pressure of the vapor present at equilibrium in a closed container of liquid • vapor pressure of a liquid depends on intermolecular forces 24 molecules = high pressure – Liquids with high vapor pressures are called volatile (evaporate readily) 12 molecules = low pressure


Concept check WHY DO LIQUIDS WITH HIGH INTERMOLECULAR FORCES HAVE A LOW VAPOR PRESSURE?

Answer

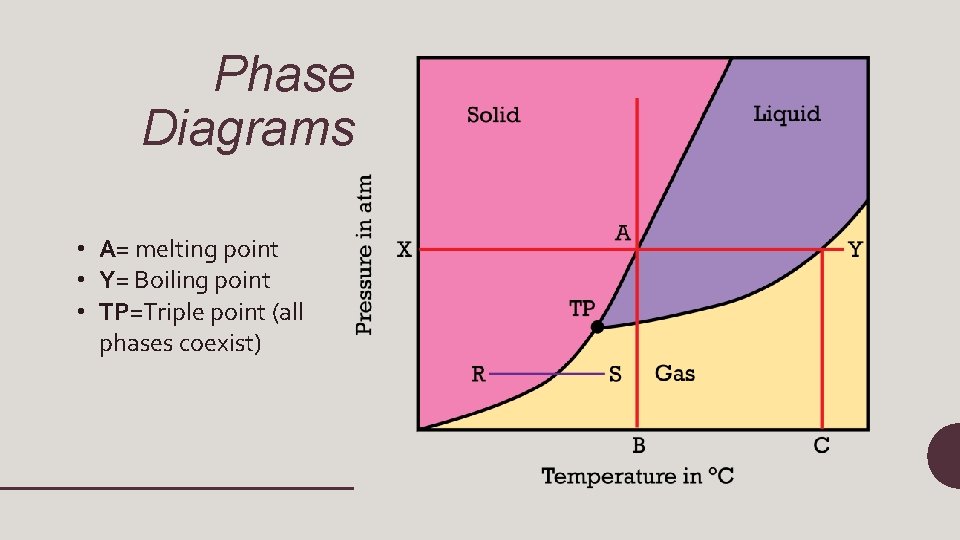
Phase Diagrams • A= melting point • Y= Boiling point • TP=Triple point (all phases coexist)

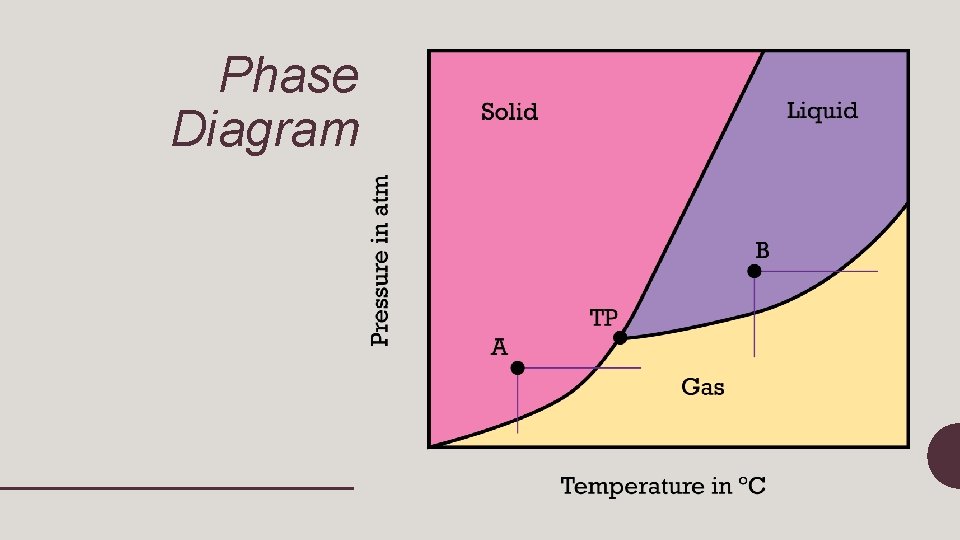
Phase Diagram

PROPERTIES OF GAS

Opening thoughts… Have you ever: Seen a hot air balloon? Had a soda bottle spray all over you? Baked (or eaten) a nice, fluffy cake? These are all examples of gases at work!

Behavior of gases are based on the following: • Pressure – Volume • Amount – Temperature

Pressure • Force that the gas exerts on a given area of the container in which it is contained. The SI unit for pressure is the Pascal, Pa OR atm. – 1 atm= 101325 Pa

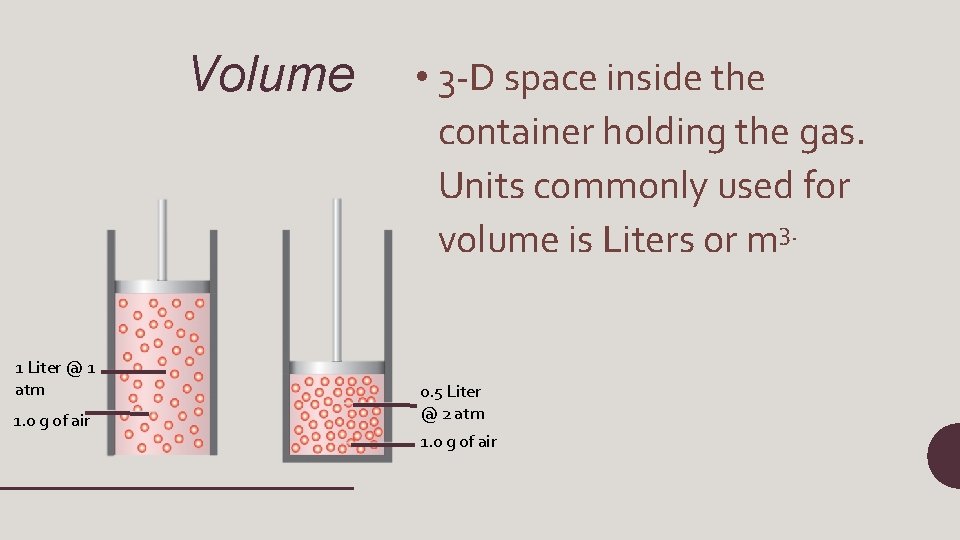
Volume 1 Liter @ 1 atm 1. 0 g of air • 3 -D space inside the container holding the gas. Units commonly used for volume is Liters or m 3. 0. 5 Liter @ 2 atm 1. 0 g of air

Amount of gas • Pumping air into a container – Increases the amount inside • Increases pressure – Double the amount of gas, double the pressure

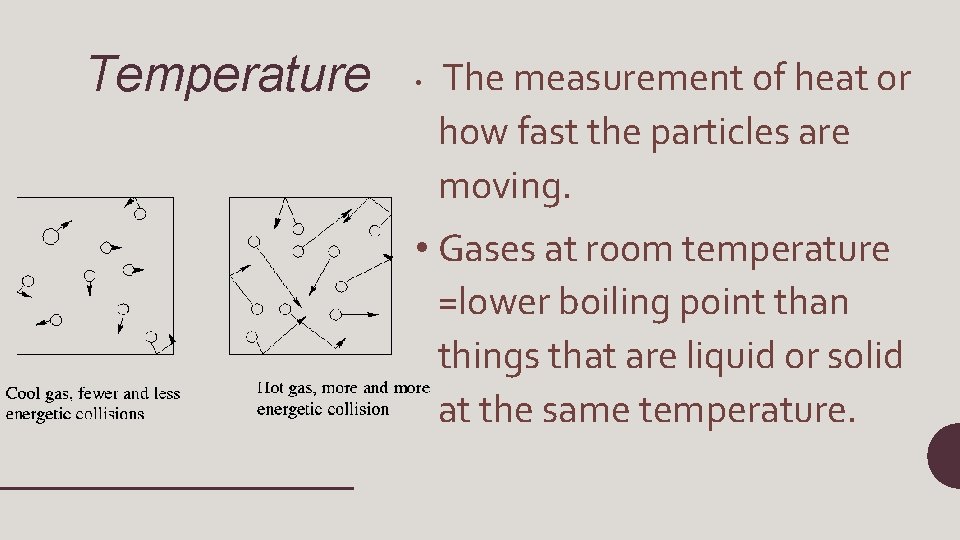
Temperature • The measurement of heat or how fast the particles are moving. • Gases at room temperature =lower boiling point than things that are liquid or solid at the same temperature.

PRESSURE AND TEMPERATURE

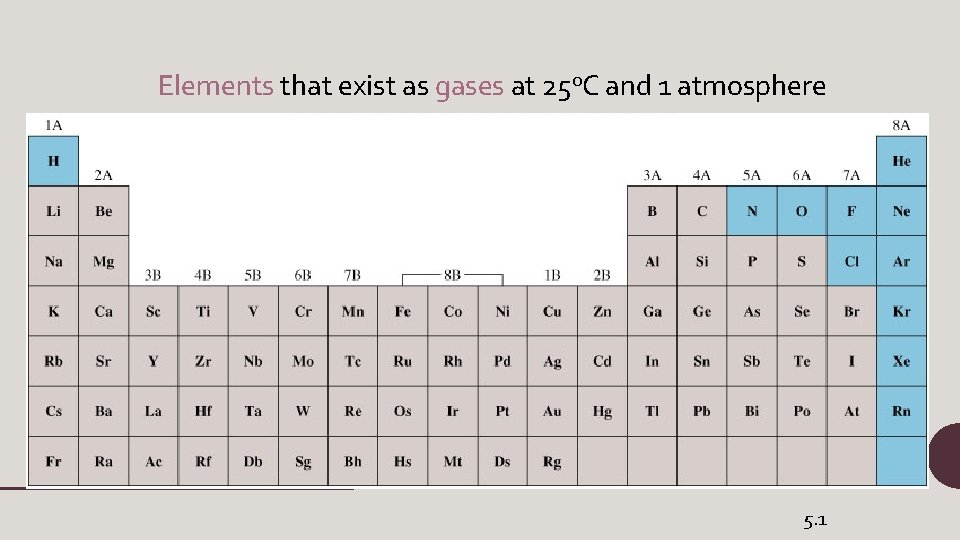
Elements that exist as gases at 250 C and 1 atmosphere 5. 1

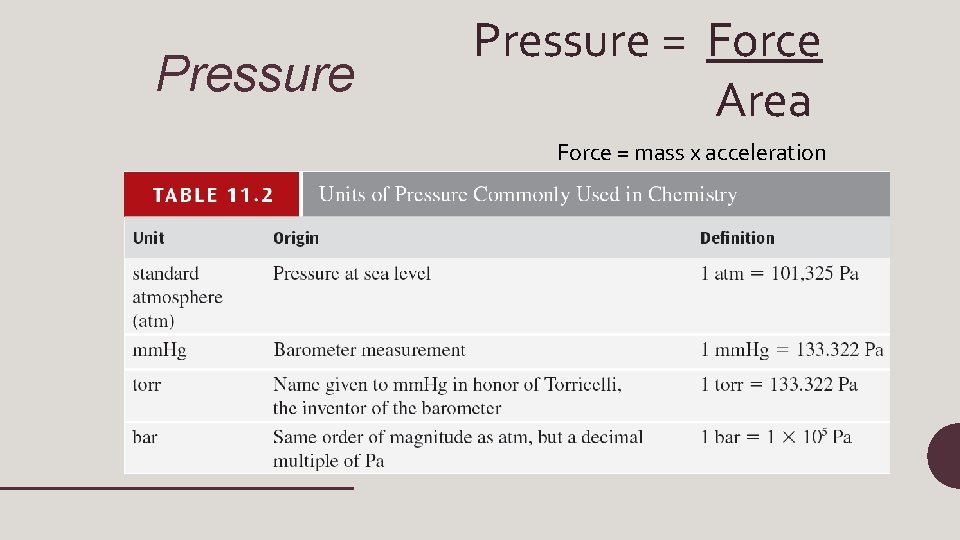
Pressure = Force Area Force = mass x acceleration

Units of pressure • 1 pascal (Pa) = 1 N/m 2 • 1 atm = 760 mm. Hg = 760 torr • 1 atm = 101, 325 Pa

1. Convert 2. 5 atm to mm. Hg 2. Convert 790 mm. Hg to atm 3. Convert 880 torr to atm

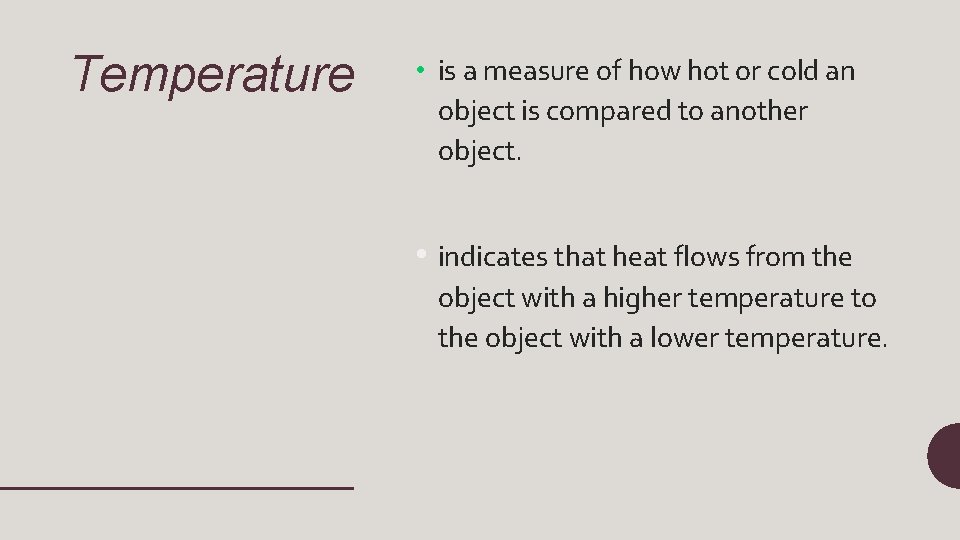
Temperature • is a measure of how hot or cold an object is compared to another object. • indicates that heat flows from the object with a higher temperature to the object with a lower temperature.

Units of temperature • 32° Fahrenheit (F) = 0 ° Celsius (C) • 0 ° Celsius (C) = 273 ° Kelvin (K)

Need to go from Celsius (C) to Kelvin (K)? • Simply add 273 to your degrees in Celsius to find your degrees in Kelvin!

THE GAS LAWS

1. Convert 2. 5 atm to mm. Hg 2. Convert 790 mm. Hg to atm 3. Convert 880 torr to atm 1 pascal (Pa) = 1 N/m 2 1 atm = 760 mm. Hg = 760 torr 1 atm = 101, 325 Pa


Gas Laws 1. Boyle’s Law 2. Charle’s Law 3. Gay-Lussac’s Law 4. Combine Gas Law 5. Ideal Gas Law

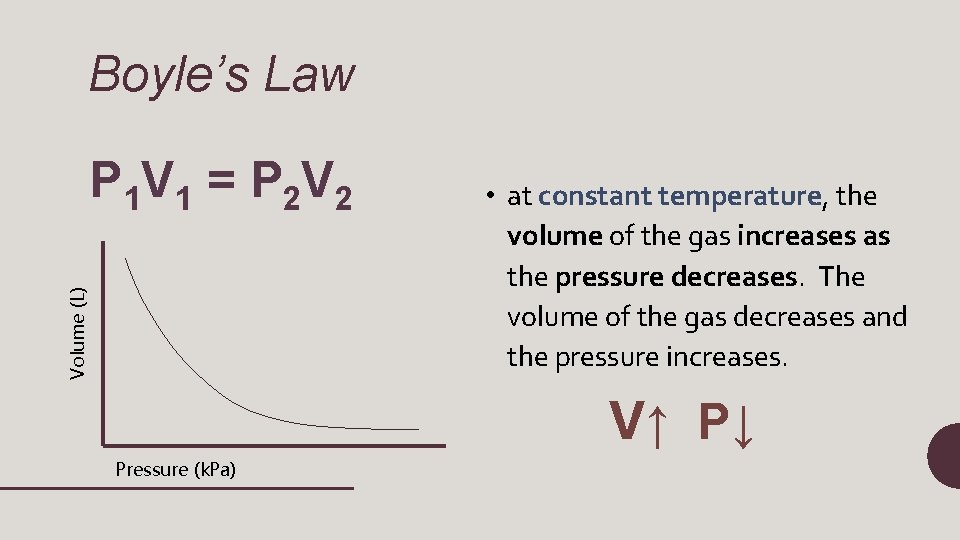
Boyle’s Law Volume (L) P 1 V 1 = P 2 V 2 • at constant temperature, the volume of the gas increases as the pressure decreases. The volume of the gas decreases and the pressure increases. V↑ P↓ Pressure (k. Pa)

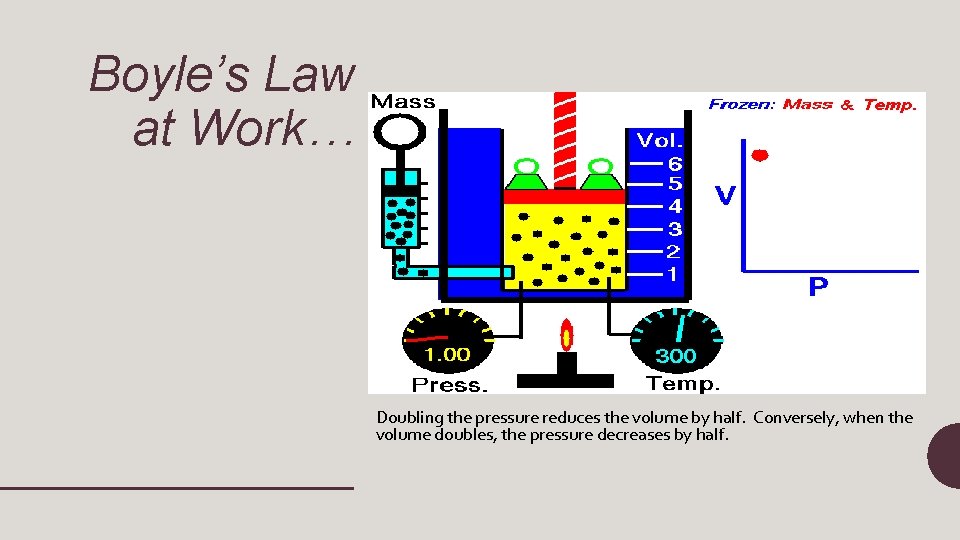
Boyle’s Law at Work… Doubling the pressure reduces the volume by half. Conversely, when the volume doubles, the pressure decreases by half.

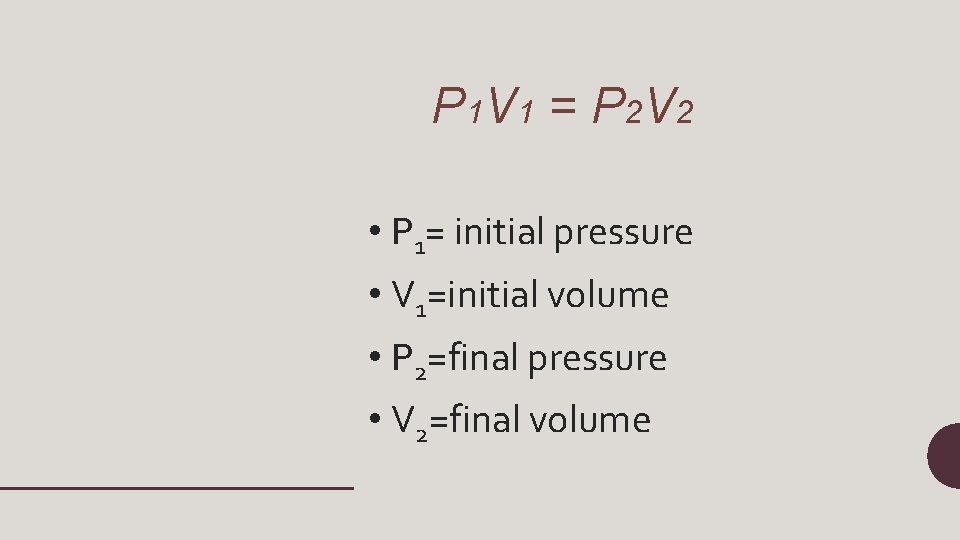
P 1 V 1 = P 2 V 2 • P 1= initial pressure • V 1=initial volume • P 2=final pressure • V 2=final volume

Practice A balloon with a volume of 2. 0 L is filled with a gas at 3 atmospheres. If the pressure is reduced to 0. 5 atmospheres without a change in temperature, what would be the volume of the balloon? (2. 0 L) (3 atm) = (0. 5 atm) (? L) P 1 V 1 = P 2 V 2

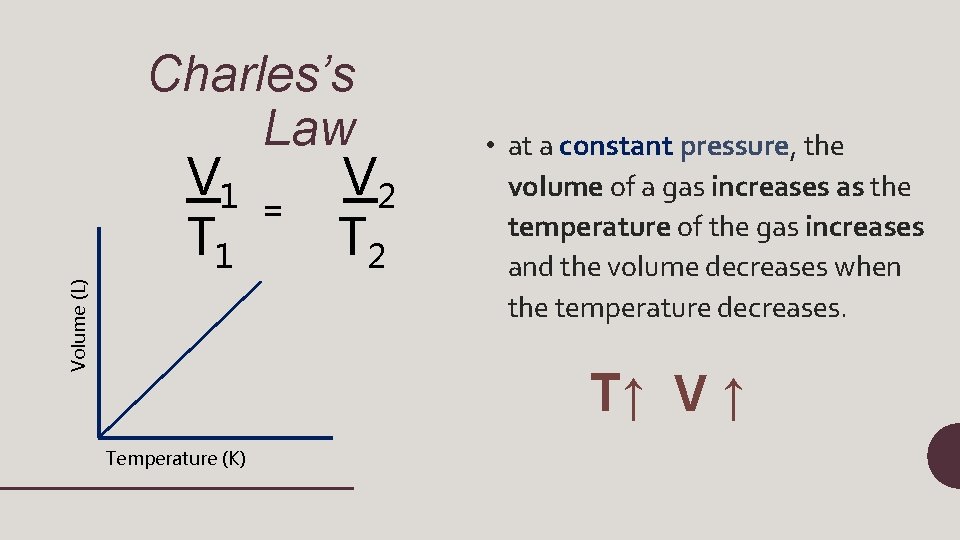
Volume (L) Charles’s Law V 1 V 2 = T 1 T 2 • at a constant pressure, the volume of a gas increases as the temperature of the gas increases and the volume decreases when the temperature decreases. T↑ V ↑ Temperature (K)

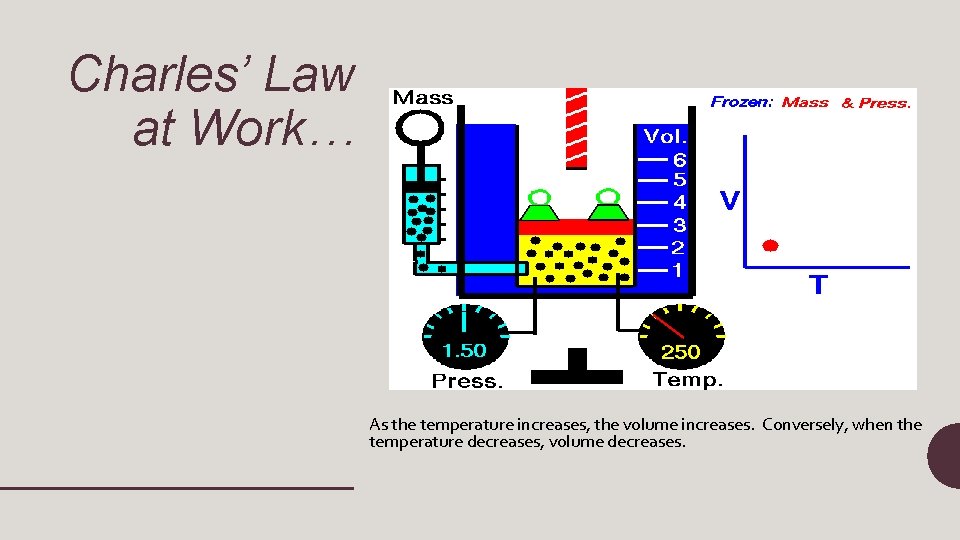
Charles’ Law at Work… As the temperature increases, the volume increases. Conversely, when the temperature decreases, volume decreases.

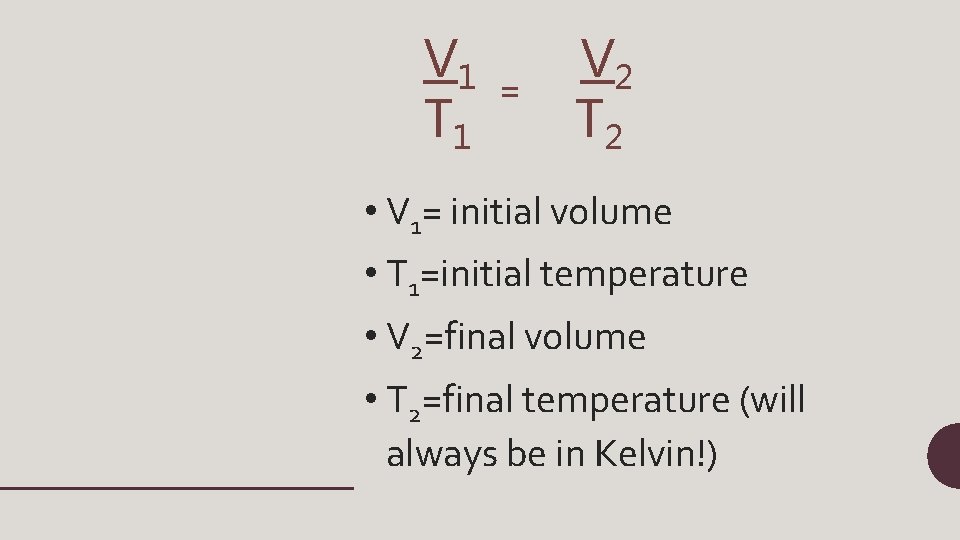
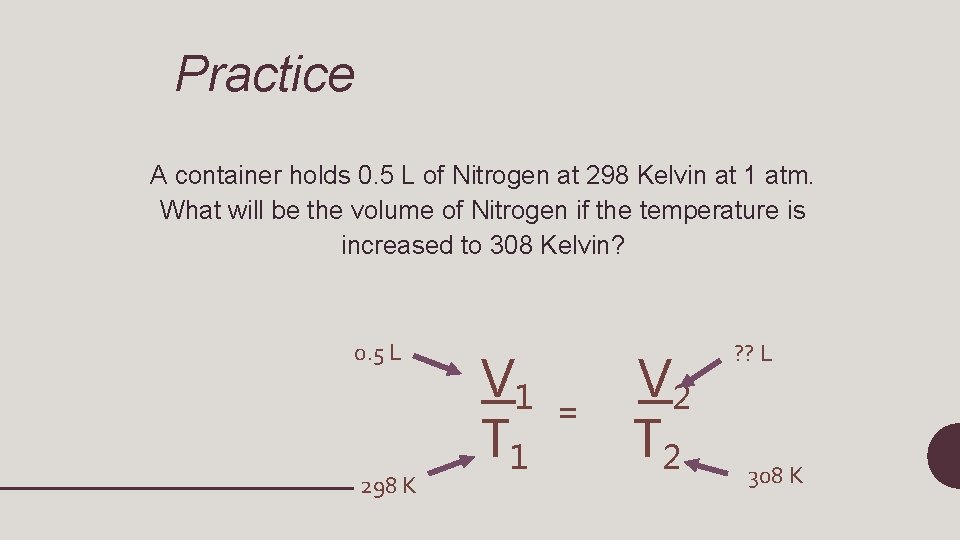
V 1 T 1 = V 2 T 2 • V 1= initial volume • T 1=initial temperature • V 2=final volume • T 2=final temperature (will always be in Kelvin!)

Practice A container holds 0. 5 L of Nitrogen at 298 Kelvin at 1 atm. What will be the volume of Nitrogen if the temperature is increased to 308 Kelvin? 0. 5 L 298 K V 1 T 1 = V 2 T 2 ? ? L 308 K
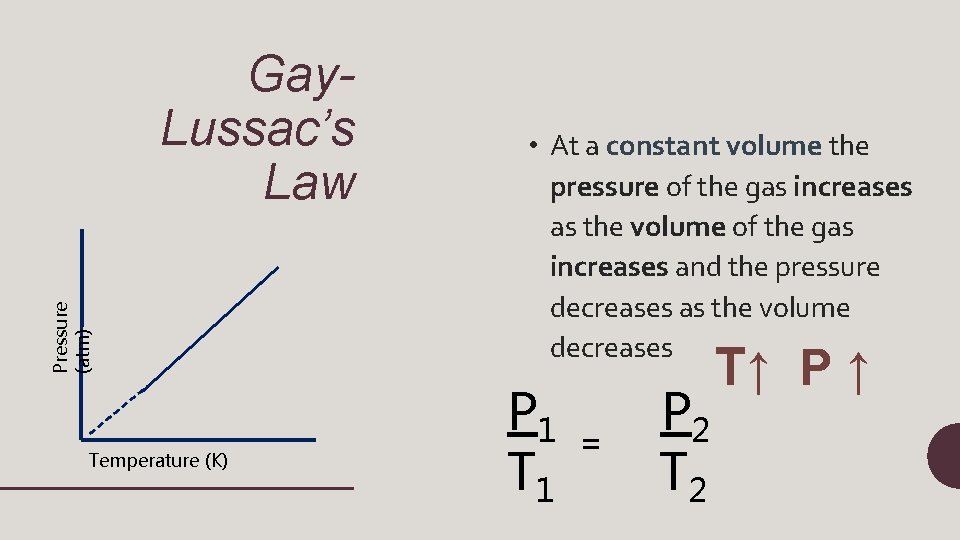
Pressure (atm) Gay. Lussac’s Law Temperature (K) • At a constant volume the pressure of the gas increases as the volume of the gas increases and the pressure decreases as the volume decreases P 1 T 1 = P 2 T↑ P ↑

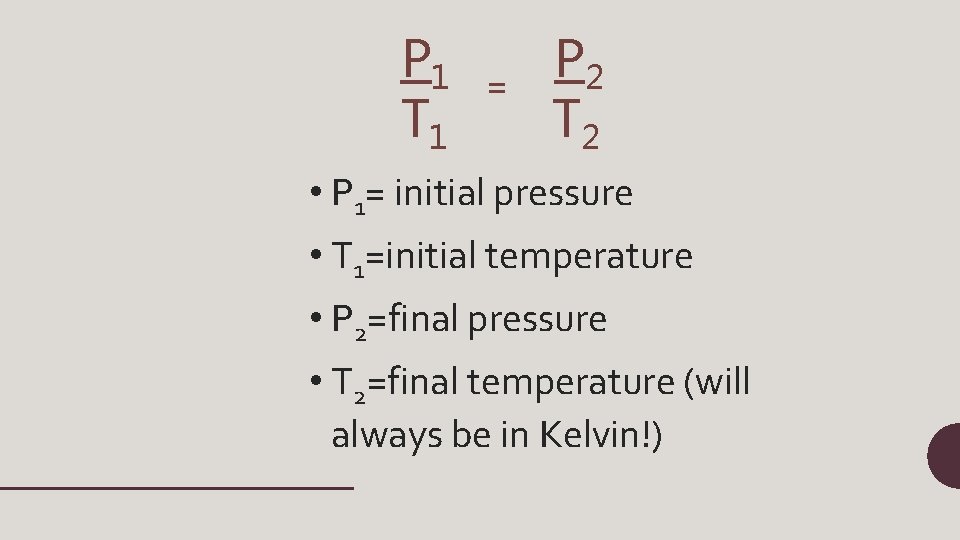
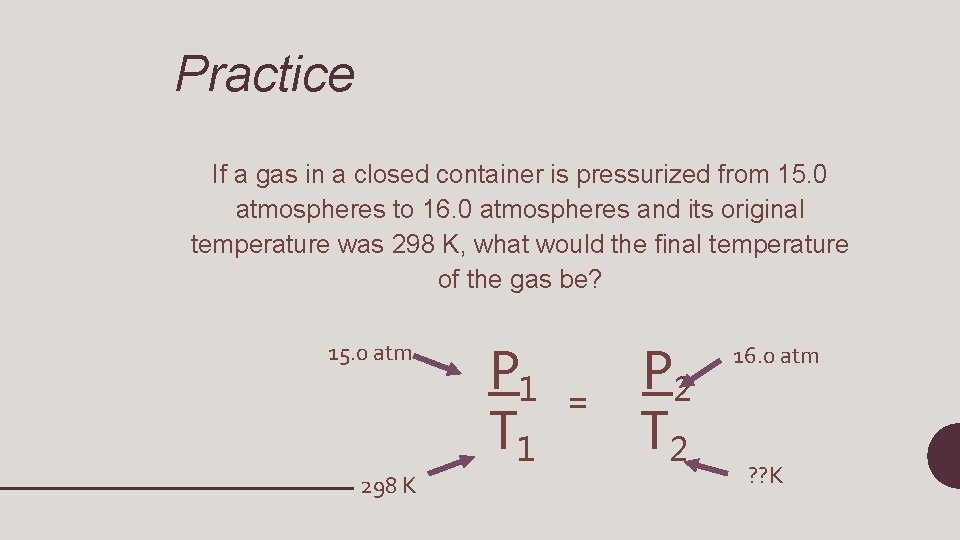
P 1 T 1 = P 2 T 2 • P 1= initial pressure • T 1=initial temperature • P 2=final pressure • T 2=final temperature (will always be in Kelvin!)

Practice If a gas in a closed container is pressurized from 15. 0 atmospheres to 16. 0 atmospheres and its original temperature was 298 K, what would the final temperature of the gas be? 15. 0 atm 298 K P 1 T 1 = P 2 T 2 16. 0 atm ? ? K

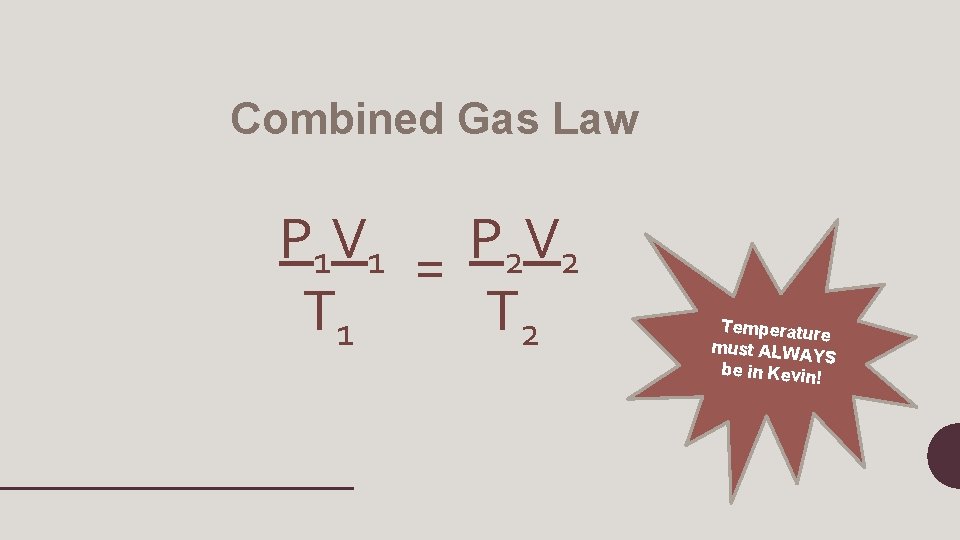
Combined Gas Law P 1 V 1 = P 2 V 2 T 1 T 2 Temperature must ALWAY S be in Kevin!

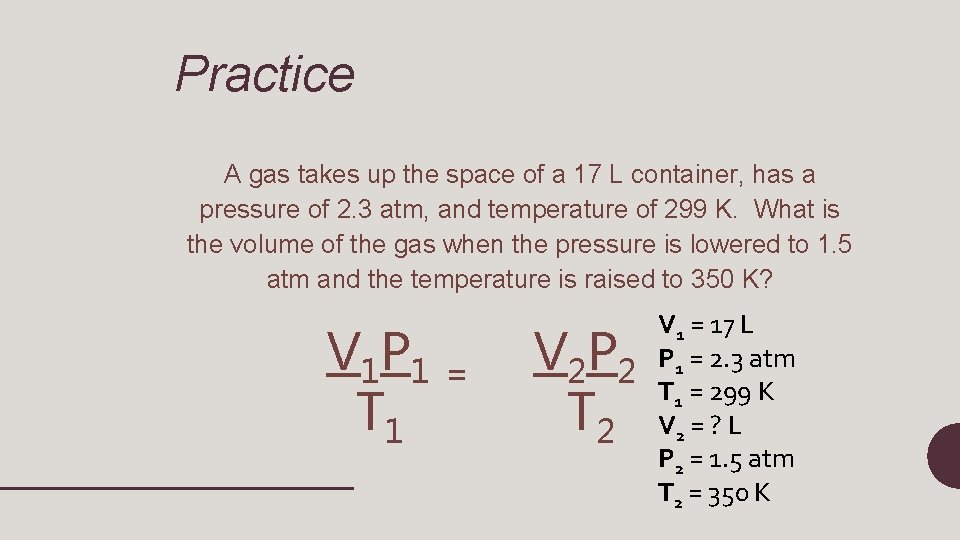
Practice A gas takes up the space of a 17 L container, has a pressure of 2. 3 atm, and temperature of 299 K. What is the volume of the gas when the pressure is lowered to 1. 5 atm and the temperature is raised to 350 K? V 1 P 1 T 1 = V 1 = 17 L P 1 = 2. 3 atm 2 2 T 1 = 299 K 2 V 2 = ? L P 2 = 1. 5 atm T 2 = 350 K VP T


THE IDEAL GAS LAW

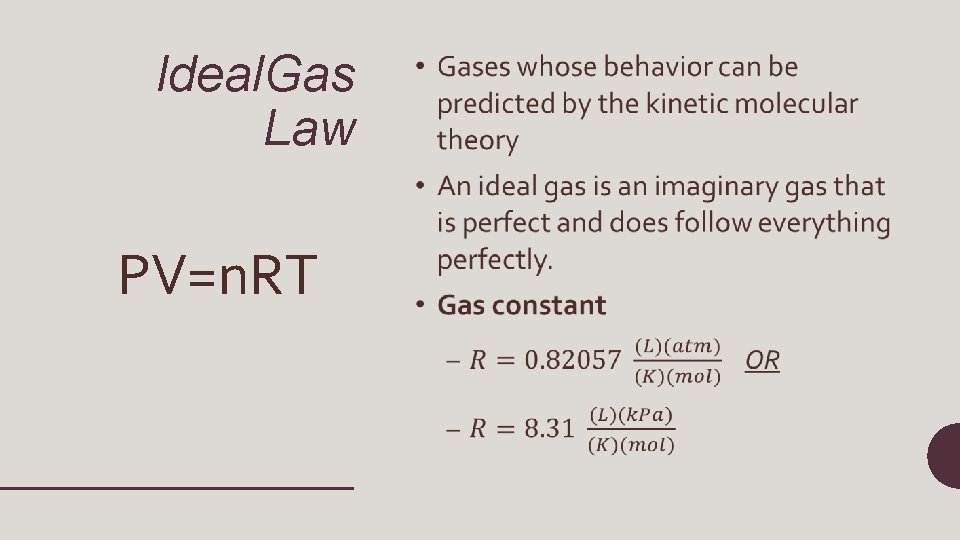
Ideal Gases • An ideal gas does not condense to a liquid at low temperatures • An ideal gas does not have forces of attraction or repulsion between particles • An ideal gas is composed of particles that have no volume

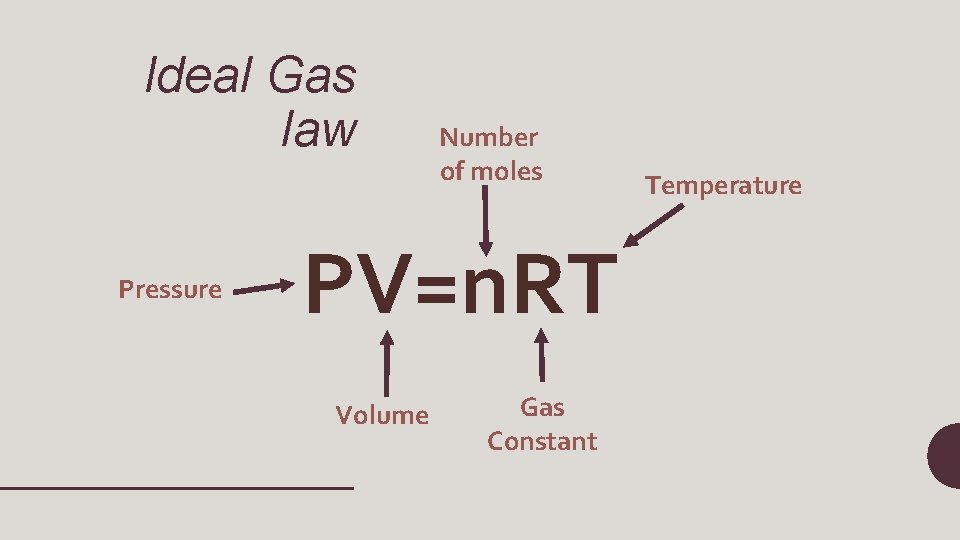
Ideal. Gas Law PV=n. RT •

Ideal Gas law Pressure Number of moles PV=n. RT Volume Gas Constant Temperature


Practice 4 moles of a gas is at a pressure of 4. 5 atm and a volume of 12 Liters. What is the temperature?

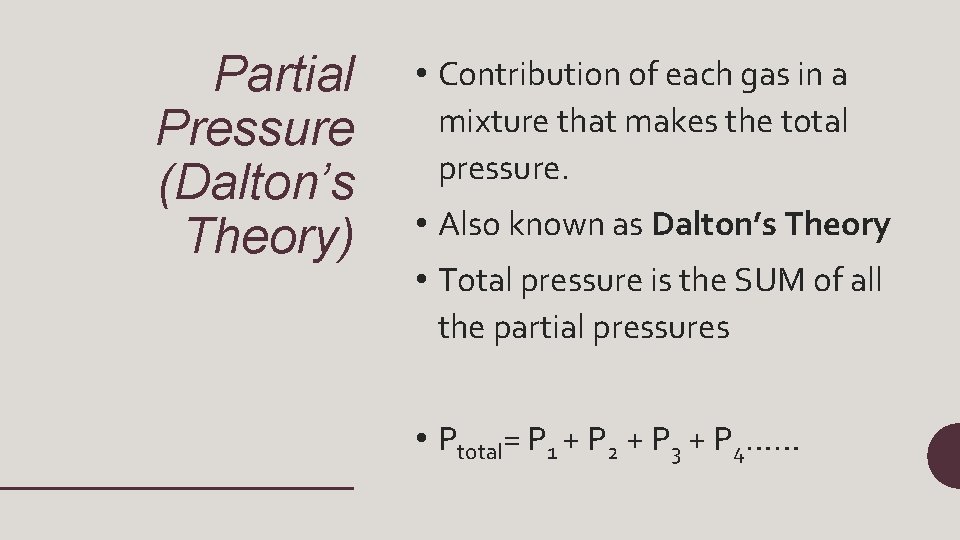
Partial Pressure (Dalton’s Theory) • Contribution of each gas in a mixture that makes the total pressure. • Also known as Dalton’s Theory • Total pressure is the SUM of all the partial pressures • Ptotal= P 1 + P 2 + P 3 + P 4……
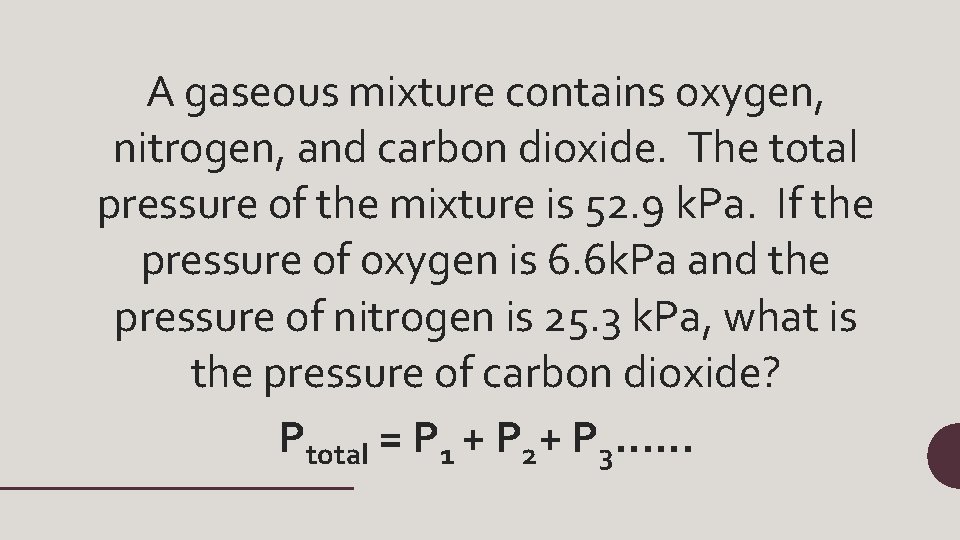
A gaseous mixture contains oxygen, nitrogen, and carbon dioxide. The total pressure of the mixture is 52. 9 k. Pa. If the pressure of oxygen is 6. 6 k. Pa and the pressure of nitrogen is 25. 3 k. Pa, what is the pressure of carbon dioxide? Ptotal = P 1 + P 2+ P 3……