States of Matter Kinetic Molecular Theory What do









- Slides: 20

States of Matter & Kinetic Molecular Theory

What do you already know?

Look Around The Room…. l l l Take a moment look around the room! Can you see things that are solid, liquid or gas? Find some, then share with the person next to you and write them down on your paper

States of Matter l l Matter is anything that has mass and volume Volume is the amount of space taken up by an object/substance l How do we measure volume? l Usually in m. L or L

States of Matter Cont’d l Mass is the amount of matter in an object/substance l The more matter in an object, the more mass it has! l How do we measure mass? l Usually in g or kg

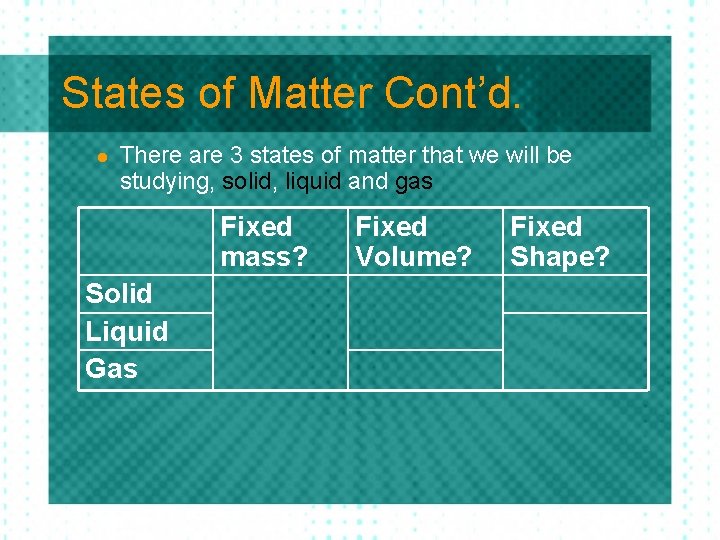
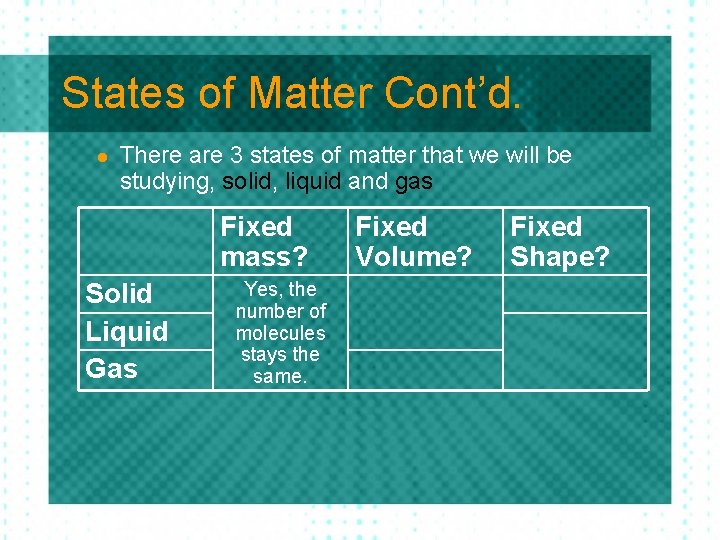
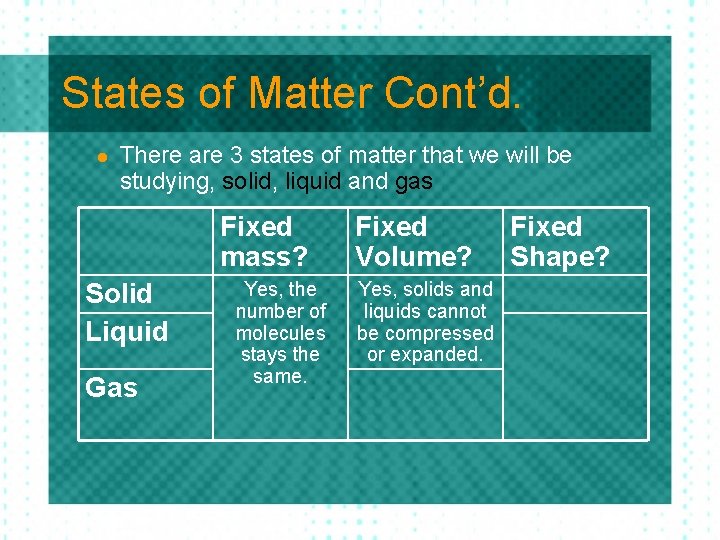
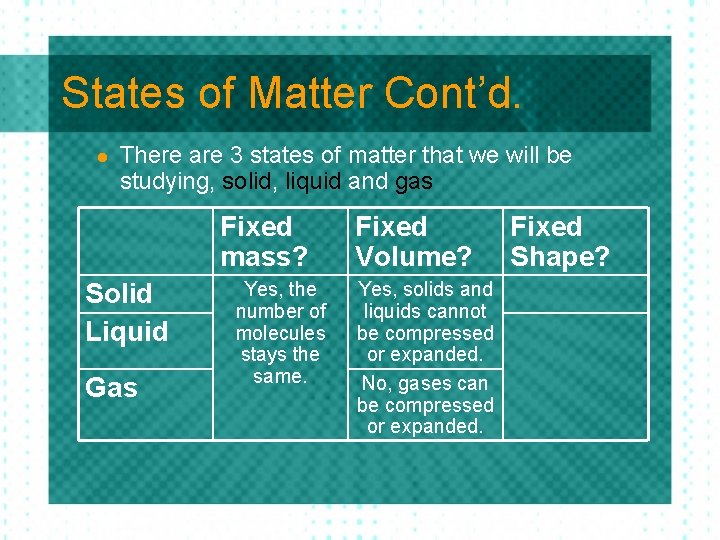
States of Matter Cont’d. l There are 3 states of matter that we will be studying, solid, liquid and gas Fixed mass? Solid Liquid Gas Fixed Volume? Fixed Shape?

States of Matter Cont’d. l There are 3 states of matter that we will be studying, solid, liquid and gas Fixed mass? Solid Liquid Gas Yes, the number of molecules stays the same. Fixed Volume? Fixed Shape?

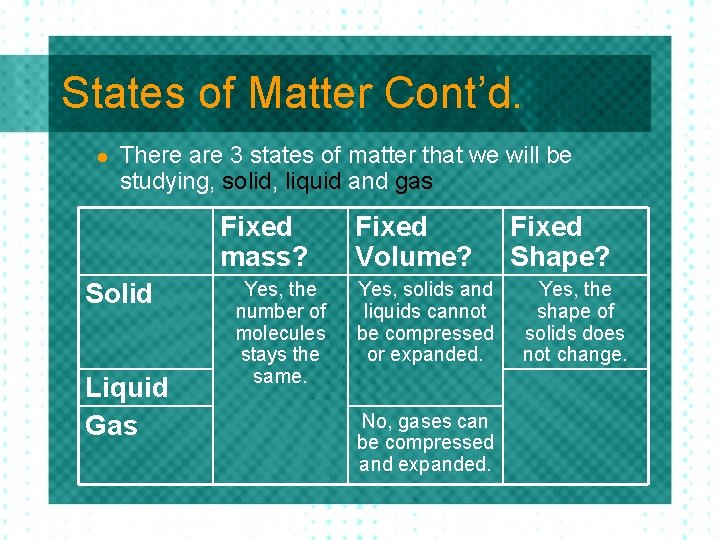
States of Matter Cont’d. l There are 3 states of matter that we will be studying, solid, liquid and gas Fixed mass? Solid Liquid Gas Yes, the number of molecules stays the same. Fixed Volume? Yes, solids and liquids cannot be compressed or expanded. Fixed Shape?

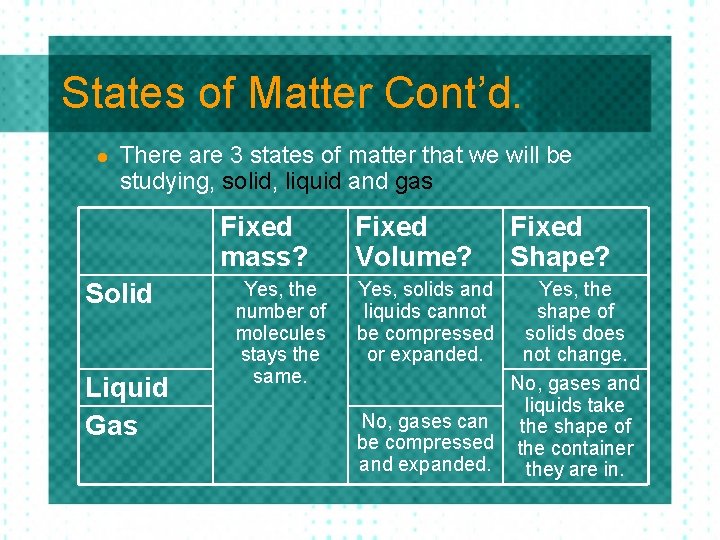
States of Matter Cont’d. l There are 3 states of matter that we will be studying, solid, liquid and gas Fixed mass? Solid Liquid Gas Yes, the number of molecules stays the same. Fixed Volume? Yes, solids and liquids cannot be compressed or expanded. No, gases can be compressed or expanded. Fixed Shape?

States of Matter Cont’d. l There are 3 states of matter that we will be studying, solid, liquid and gas Fixed mass? Solid Liquid Gas Yes, the number of molecules stays the same. Fixed Volume? Yes, solids and liquids cannot be compressed or expanded. No, gases can be compressed and expanded. Fixed Shape? Yes, the shape of solids does not change.

States of Matter Cont’d. l There are 3 states of matter that we will be studying, solid, liquid and gas Fixed mass? Solid Liquid Gas Yes, the number of molecules stays the same. Fixed Volume? Yes, solids and liquids cannot be compressed or expanded. Fixed Shape? Yes, the shape of solids does not change. No, gases and liquids take No, gases can the shape of be compressed the container and expanded. they are in.

Paddle Time! l Identifying solids, liquids, and gases

Video l https: //www. youtube. com/watch? v=1 Jtw 8 g 795 Us

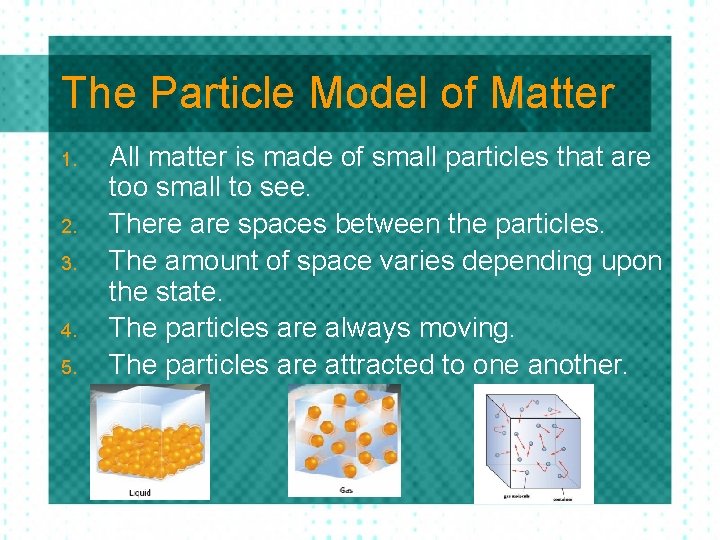
The Particle Model of Matter 1. 2. 3. 4. 5. All matter is made of small particles that are too small to see. There are spaces between the particles. The amount of space varies depending upon the state. The particles are always moving. The particles are attracted to one another.

Kinetic Molecular Theory l The Kinetic Molecular Theory (KMT) explains what happens to matter when the kinetic energy of particles changes. l What is Kinetic energy? l Kinetic energy is the energy due to motion

Kinetic Molecular Theory l Particles are always moving, in gases, liquids and even solids! This means they have kinetic energy!!

The Kinetic Molecular Theory 1. All matter is made of very small particles. 2. There is empty space between particles. 3. Particles are constantly moving. The particles are colliding with each other and the walls of their container. 4. Energy makes particles move. The more energy the particles have, the faster they move and further apart they get.

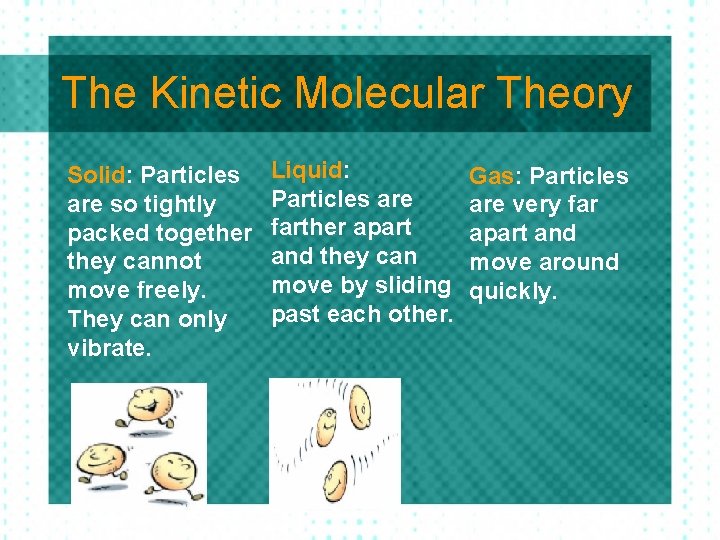
The Kinetic Molecular Theory Solid: Particles are so tightly packed together they cannot move freely. They can only vibrate. Liquid: Particles are farther apart and they can move by sliding past each other. Gas: Particles are very far apart and move around quickly.

Practice l Do the worksheet on the back of your notes package

Demo Time! l Cool Contractions Demo