States of Matter Intro to Energy Textbook Chapt

States of Matter & Intro to Energy Textbook Chapt 13 Review Book Topic 4 (Partially)

What’s the difference between temperature and heat? Are they the same thing?

Temperature § The temperature of a substance is a measure of the average kinetic energy of its particles.
Temperature § The temperature difference between two bodies indicates the direction of heat flow. § Heat flows from an object with higher temp to an object with lower temp (until they reach the same temp)

Temperature § The average kinetic energy depends only on the temperature of the substance, not on the nature or the amount of material. § So, 10 g of water @ 500 C has greater average kinetic energy than 600 g of iron at 200.

Temperature § Temperature is measured using a thermometer § Thermometers are calibrated using two fixed reference points § freezing point of water (solid-liquid equilibrium) § boiling point of water (liquid-gas equilibrium
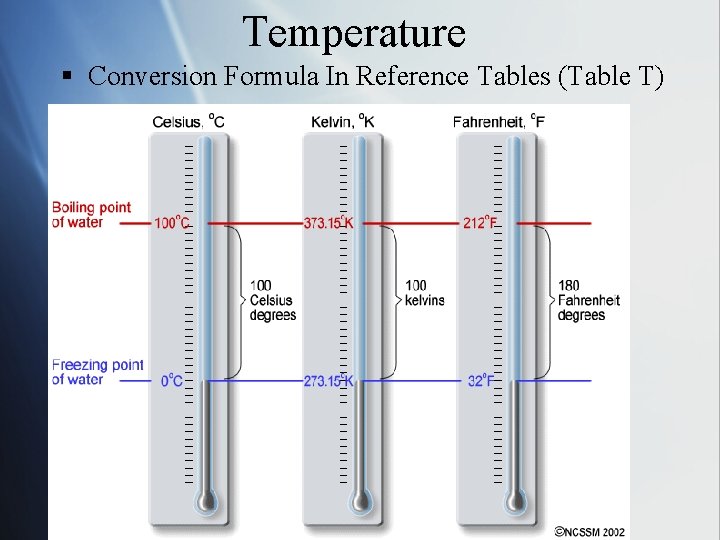
Temperature § Conversion Formula In Reference Tables (Table T)
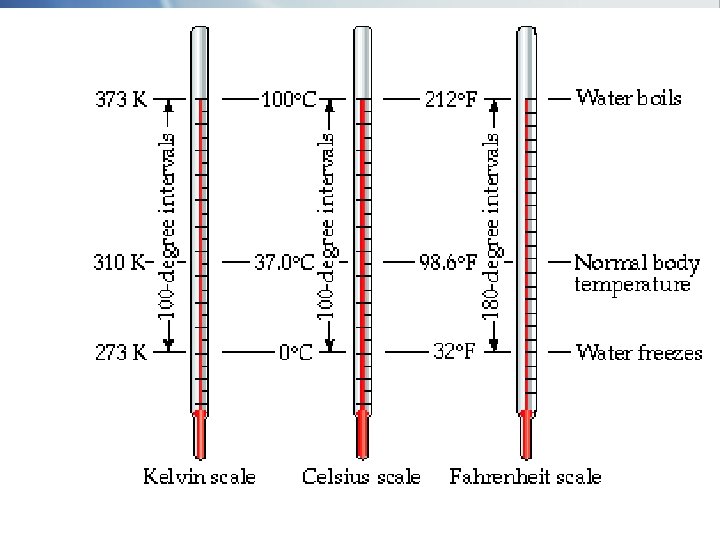

Celsius and Kelvin Temperature Scales § The Celsius and Kelvin scales are related by the equation K = 0 C + 273 (ref. tables)

Temperature Vs. Heat § Heat and temperature are not the same. § Heat is a measure of the amount of energy transferred from one substance to another.

Video - Bill Nye: Heat and Energy Video Worksheet

Chemistry Lab Endothermic and Exothermic Reactions Intro to thermo chemical equations

Endothermic Process § Processes that require energy in order to occur. §Breaking a bond is an endothermic process

Exothermic Process § Processes that release thermal energy when they occur § Making a bond is an exothermic process

Phase Changes Endothermic and Exothermic “processes” includes chemical reactions (as in your lab), and also includes phase changes.

Phase Changes § Phase Changes that require energy § Melting - changing from a solid to a liquid § Vaporization - changing from a liquid to a gas § Sublimation - changing from a solid to a gas (without first becoming a liquid)

Phase Changes § Phase Changes That Release Energy § Condensation - changing from a vapor to a liquid § Freezing - changing from a liquid to a solid § Deposition - changing from a vapor to a solid (without becoming a liquid first)

Chemistry Lab Freezing/Melting of Water
Kinetic-Molecular Theory §Kinetic Molecular Theory (KMT) is a model used to explain the behavior of gases.
Major Ideas of Kinetic Molecular Theory § Gases contain particles (usually molecules or atoms) that are in constant, random, straight line motion.
Major Ideas of Kinetic Molecular Theory § Gas particles collide with each other and with the walls of the container. The collisions transfer energy, but there is no net loss of energy.

Major Ideas of Kinetic Molecular Theory § Gas particles are separated by relatively great distances. Because of this, the volume occupied by the particles themselves is negligible.

Major Ideas of Kinetic Molecular Theory § Gas particles do not attract each other.

Jigsaw Activity Ch 13 Sections 1 and 2

Liquids § When applying KMT to liquids, we must now consider the intermolecular forces of attraction.

Liquids § Forces of attraction between liquid particles limit their range of motion so they remain closely packed in a fixed volume.

Liquids § The higher density of liquids must be traced to the intermolecular forces that hold particles together.

Viscosity § The viscosity of a liquid is determined by the type of IMF’s involved, the shape of the particles, and the temperature.

Surface Tension § IMF’s do not have an equal effect on all particles in a liquid. § For particles at the surface, there are no attractions from above to balance the attractions from below.

Solids § Most solids are more dense than their liquid counterparts §Exception is water

Solids - Strong IMF’s § Crystalline Solid - atoms, ions, or molecules are arranged in an orderly, three dimensional structure.
Challenge Question § Say I heat up some water from 100 C to 250 C. What factors determine how much heat is necessary to do so?

§ The amount of heat given off or absorbed in a reaction can be calculated using the following equation: q = m. C T (reference tables) Where: q = heat (Joules) m = mass of the substance (grams) C = specific heat capacity of the substance (J/g • 0 C) T = (initial temperature - final temperature)

Sample Problem § When 10 grams of water are cooled from 80. 00 C to 70. 00 C, What is the number of joules of heat energy released?

Heat of Fusion § The amount of heat needed to convert a unit mass of a substance from solid to liquid is called the heat of fusion.

Heat of Fusion q = m Hf (ref tables) Where: q = heat m = mass Hf = heat of fusion Heat of fusion indicates how strong/weak the IMF’s are.

Sample Problem § How many joules of heat energy would need to be absorbed to completely 0 melt 100 g of ice at 0 C?

Heat of Vaporization § The amount of heat needed to convert a one gram of a substance from its liquid phase to its vapor phase at constant temperature is called its heat of vaporization. § Heat of vaporization is an indication of how strong the IMF’s are.

Heat of Vaporization q = m Hv (ref tables) Where: q = heat m = mass Hv = heat of vaporization

Sample Problem § How many joules of energy are required to vaporize 200 g of water at 0 100 C and 1 atm?
- Slides: 40