States of Matter How is the particle diagram




- Slides: 8

States of Matter How is the particle diagram of a solid different from one of a gas?

Quick question: • Using your ipads, find the two characteristics of a liquid. What did you find? A liquid has a definite volume and no definite shape, so your can pour a liquid in different shaped objects and that liquid will become that shape. So now, I have a new question…

Is a cat a liquid? • Marc-Antoine Fardin, a French physicist, WON the Nobel Peace Prize in 2017 in Improbable Research (mixing humor and science) for answering this question.

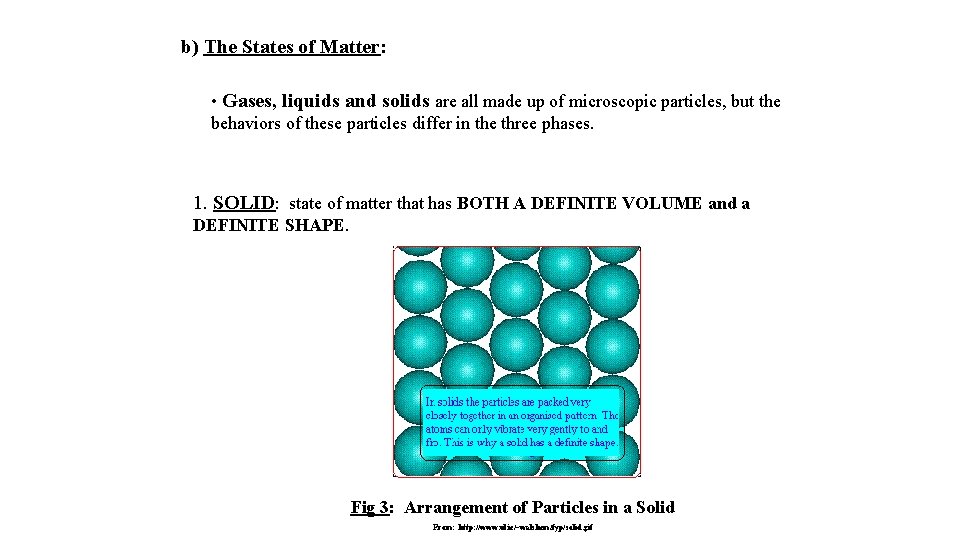
b) The States of Matter: • Gases, liquids and solids are all made up of microscopic particles, but the behaviors of these particles differ in the three phases. 1. SOLID: state of matter that has BOTH A DEFINITE VOLUME and a DEFINITE SHAPE. Fig 3: Arrangement of Particles in a Solid From: http: //www. ul. ie/~walshem/fyp/solid. gif

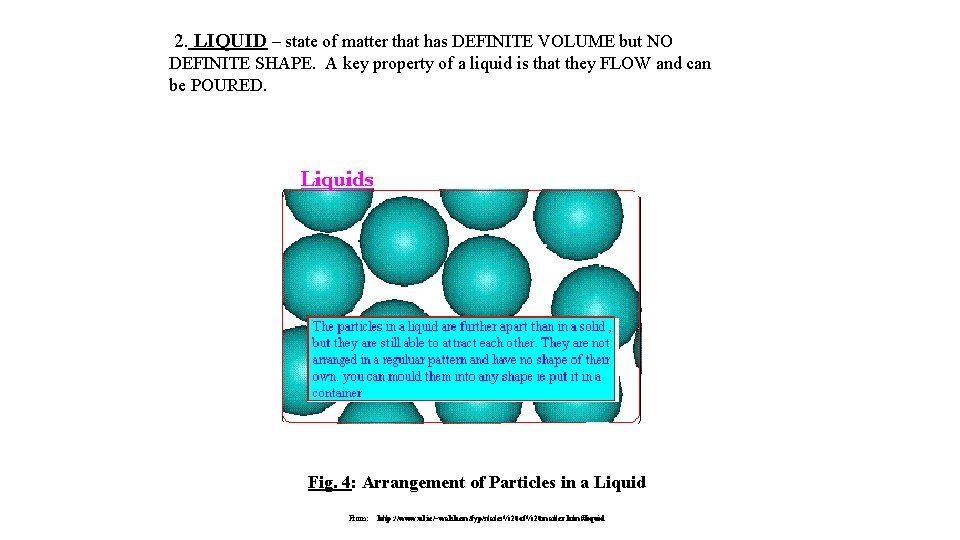
2. LIQUID – state of matter that has DEFINITE VOLUME but NO DEFINITE SHAPE. A key property of a liquid is that they FLOW and can be POURED. Fig. 4: Arrangement of Particles in a Liquid From: http: //www. ul. ie/~walshem/fyp/states%20 of%20 matter. htm#liquid

3. GAS – state of matter that has NO DEFINITE VOLUME and NO DEFINITE SHAPE. • A Gas ALWAYS TAKES BOTH THE VOLUME AND THE SHAPE OF ANY CONTAINER INTO WHICH IT IS PLACED. If a gas is NOT in a container, it will spread out as far as it can. Fig. 5: Arrangement of Particles in a Gas From : http: //www. ul. ie/~walshem/fyp/gas. gif

Ex: #1 Under the same conditions of temperature and pressure, a liquid differs from a gas because the particles of the liquid: a) are in constant straight-line motion b) take the shape of the container they occupy c) have no regular arrangement d) have stronger forces of attraction between them

The fourth state of matter: Plasma • Plasmas are very different and unique from the other states of matter. Plasmas are very similar to gases. The key difference is where the positively and negatively charged particles are located. In neon gas, the electrons are all bound to the nucleus. In neon plasma, the electrons are free to move around the system.