States of Matter Goal of the class To







- Slides: 15

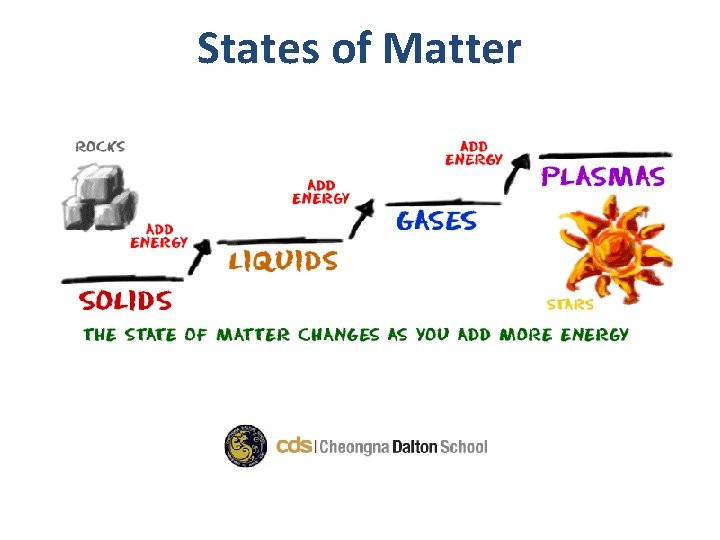
States of Matter

Goal of the class • To understand the properties of a solid, liquid and gas. • Question of the day: What is surface tension? • Previous answer: We can separate a mixture by filtration, magnetism, distillation and evaporation.

Introduction • We can all think of examples of solids, liquids and gases. • They can be elements, compounds or mixtures. • Examples include: – Solid: Gold (element) – Liquid: Water (compound & molecule) – Gas: Air (homogeneous mixture)

Solids • Imagine you had a diamond and you picked it up. Then placed it in a bowl. Would the diamond change size or shape? No, of course not • A solid has a definite shape and volume.

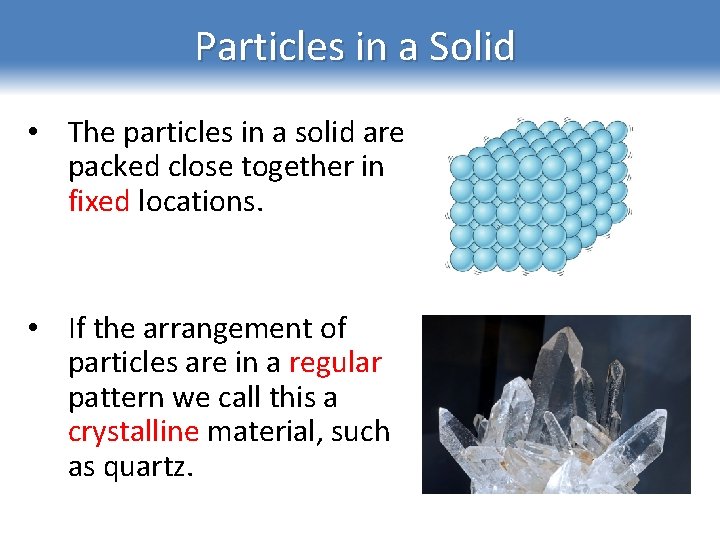
Particles in a Solid • The particles in a solid are packed close together in fixed locations. • If the arrangement of particles are in a regular pattern we call this a crystalline material, such as quartz.

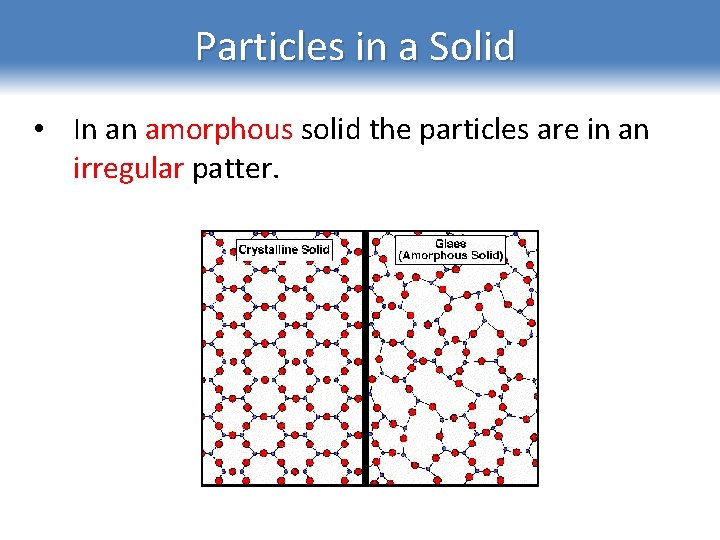
Particles in a Solid • In an amorphous solid the particles are in an irregular patter.

Liquids • A liquid has a definite volume but no fixed shape. • So if I pour 500 m. L of milk from a square milk carton into a round glass. The amount of milk is still 500 m. L, it’s just a different shape now.

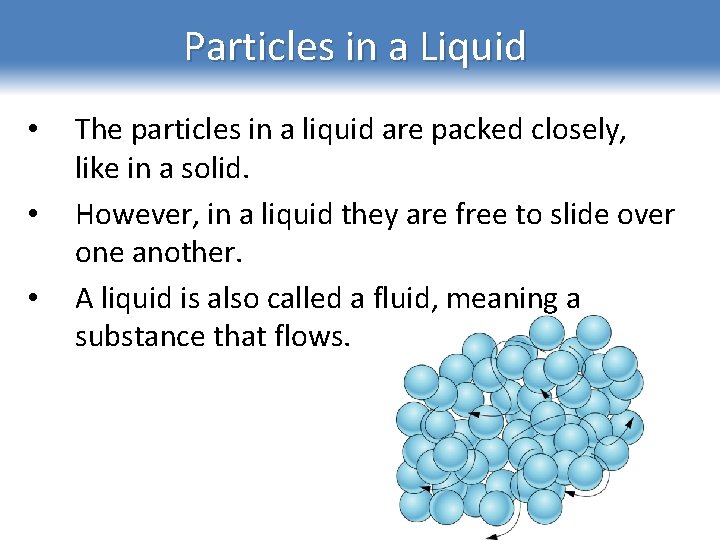
Particles in a Liquid • • • The particles in a liquid are packed closely, like in a solid. However, in a liquid they are free to slide over one another. A liquid is also called a fluid, meaning a substance that flows.

Liquid Properties • • Water and other liquids have a property called surface tension that acts like a skin. The water molecules want to be as close as to each other as they can.

Liquid Properties

Liquid Properties • What shape is a water droplet? Water droplets are spherical

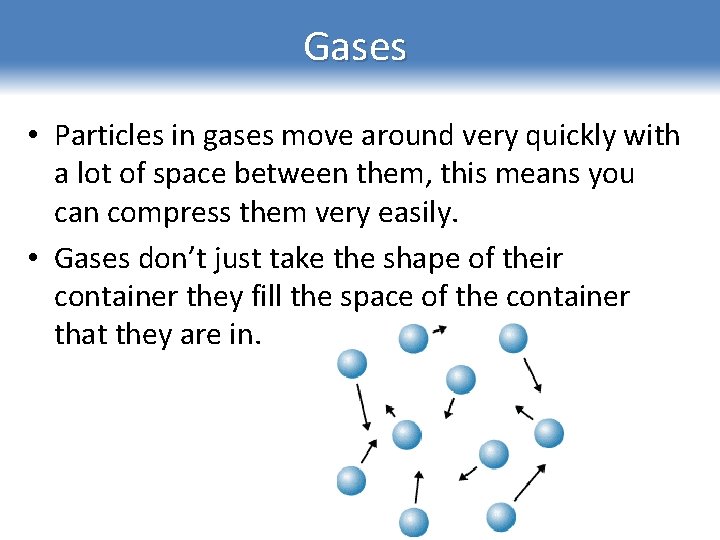
Gases • Particles in gases move around very quickly with a lot of space between them, this means you can compress them very easily. • Gases don’t just take the shape of their container they fill the space of the container that they are in.

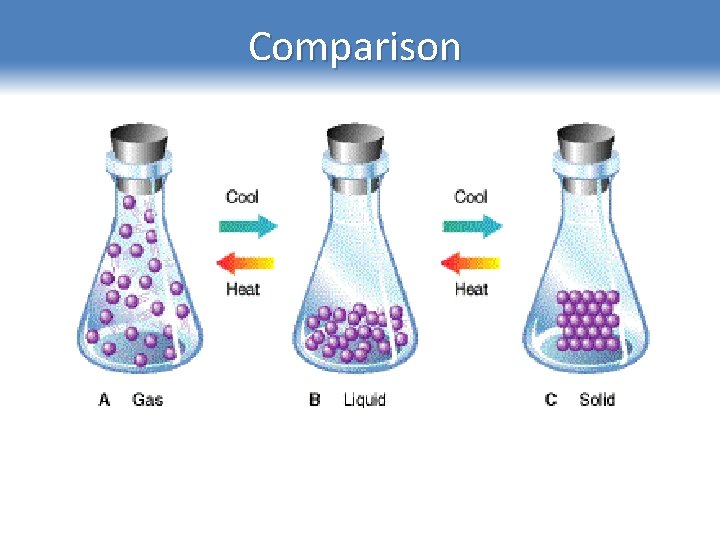
Comparison

Vocabulary • Crystal – Having a highly ordered arrangement of atoms in all directions. • Amorphous – Lacking any long range order of its structure. • Surface Tension – The ‘skin’ on a liquid from the attraction of the molecules.

Homework • Please complete Chemical Building Blocks workbook page 11