STATES OF MATTER DIFFUSION Learning Points Explain diffusion











- Slides: 17

STATES OF MATTER

DIFFUSION

Learning Points • Explain diffusion in terms of particle theory.

Diffusion If you open a bottle of perfume at arm's length you can soon smell it. Perfume particles mingle with air particles and move through the air until they reach your nose. We call this diffusion. pocketchange. become. com

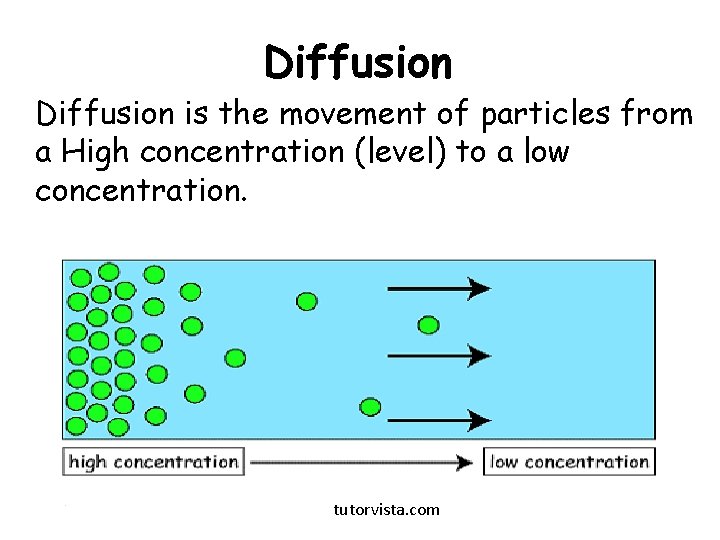
Diffusion is the movement of particles from a High concentration (level) to a low concentration. tutorvista. com

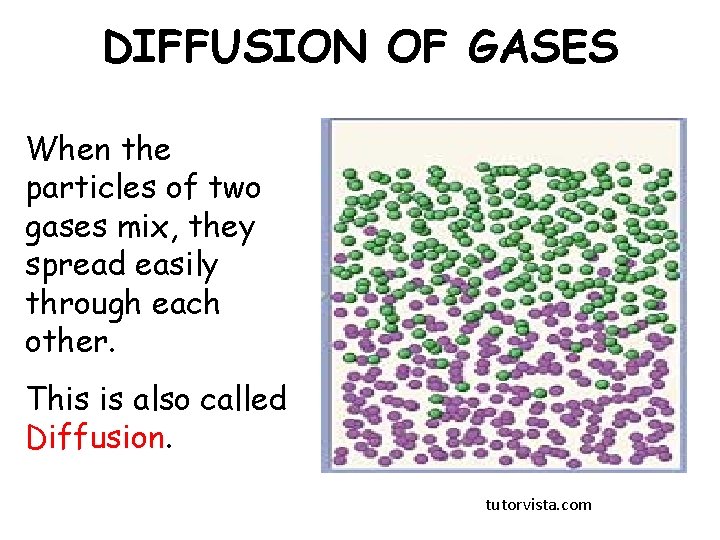
DIFFUSION OF GASES When the particles of two gases mix, they spread easily through each other. This is also called Diffusion. tutorvista. com

QUICK EXPERIMENT AIM: To find out if gases diffuse (move) through the air.

INSTRUCTIONS • Collect a stop-watch and set it to zero time. • Close your eyes and start the watch when your teacher has said that they have sprayed a substance into the classroom. • Stop the watch when you smell the substance. • Compare how long it took you to smell the gas substance.

WHAT HAPPENED? CAN YOU EXPLAIN WHY?

EXPLANATION • Pupils closest to the sprayed gas smell it more quickly than those further away, because it is more concentrated. • Different gases diffuse (move) through the air at different speeds.

DIFFUSION OF GASES Your teacher is going to show you a brown coloured gas diffusing.

DIFFUSION OF GASES Nitrogen dioxide diffusing flickr. com

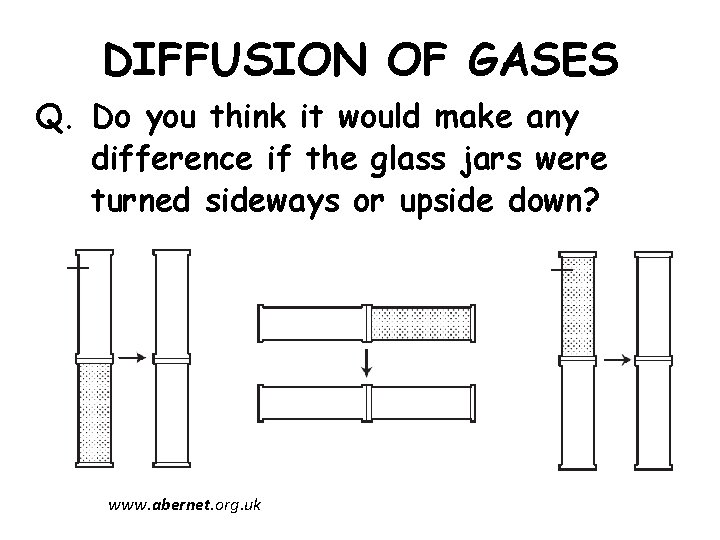
DIFFUSION OF GASES Q. Do you think it would make any difference if the glass jars were turned sideways or upside down? www. abernet. org. uk

WHY DO GASES DIFFUSE? Gas particles move from an area where there is a HIGH level to an area where there is a low LEVEL of the particles i. e. DIFFUSION.

DIFFUSION OF GASES Click here to see a video on the diffusion of gases. http: //www. youtube. com/watch? v=H 7 Qs. Ds 8 Z RMI

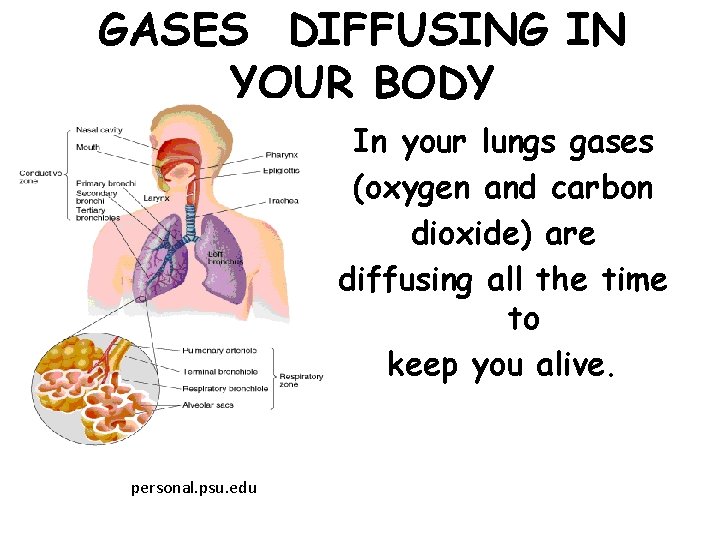
GASES DIFFUSING IN YOUR BODY In your lungs gases (oxygen and carbon dioxide) are diffusing all the time to keep you alive. personal. psu. edu

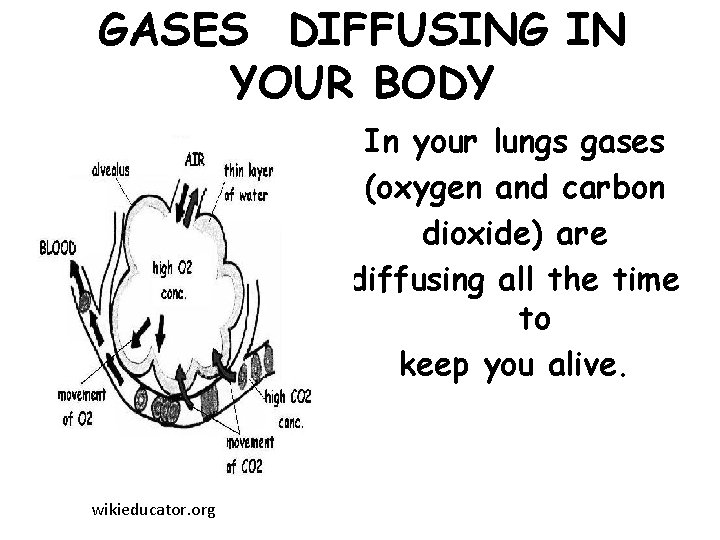
GASES DIFFUSING IN YOUR BODY In your lungs gases (oxygen and carbon dioxide) are diffusing all the time to keep you alive. wikieducator. org