STATES OF MATTER CHARACTERISTIC PROPERTIES VS NONCHARACTERISTIC PROPERTIES










- Slides: 20

STATES OF MATTER CHARACTERISTIC PROPERTIES VS NON-CHARACTERISTIC PROPERTIES
STATES OF MATTER 1 -Every substance in the world around us exists as a solid, liquid or gas. States of matter Solid (S) Liquid (L) Gas (G)

MATTER 2 - Matter is anything which occupies space and which has mass. solid liquid gas

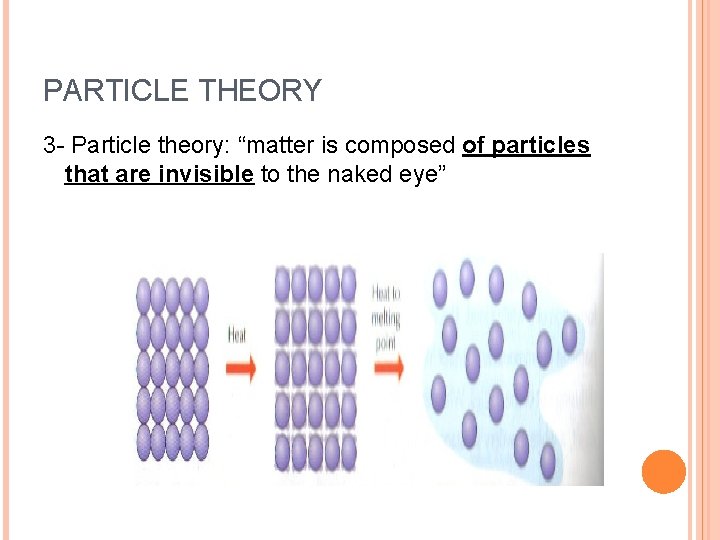
PARTICLE THEORY 3 - Particle theory: “matter is composed of particles that are invisible to the naked eye”


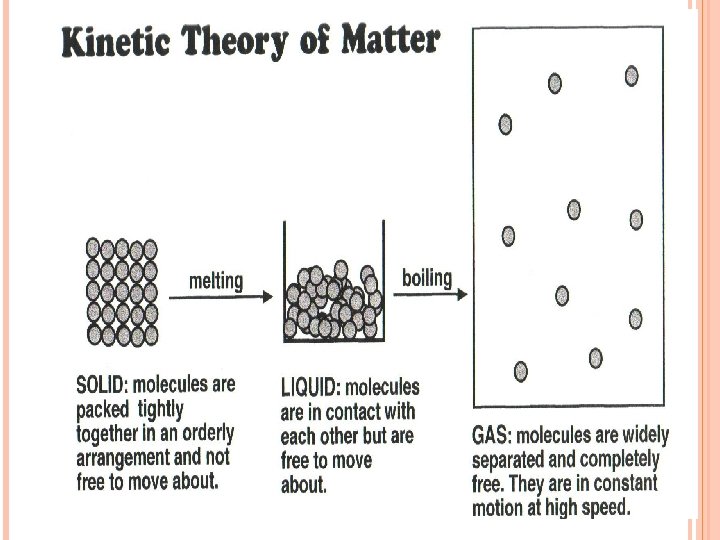
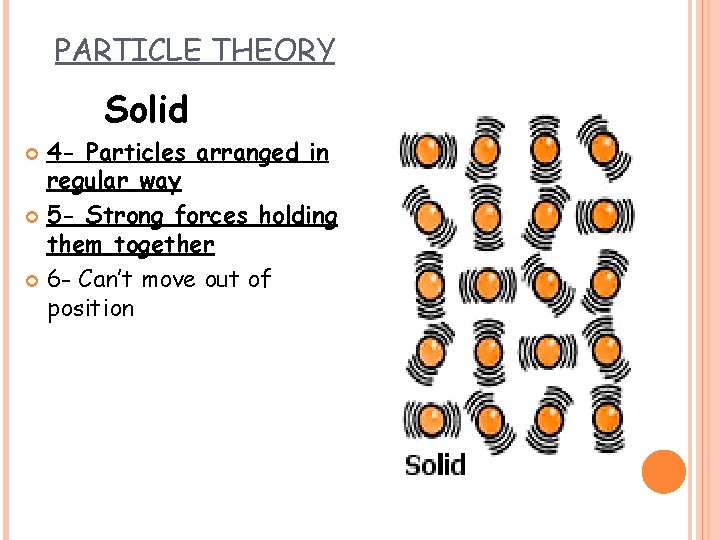
PARTICLE THEORY Solid 4 - Particles arranged in regular way 5 - Strong forces holding them together 6 - Can’t move out of position

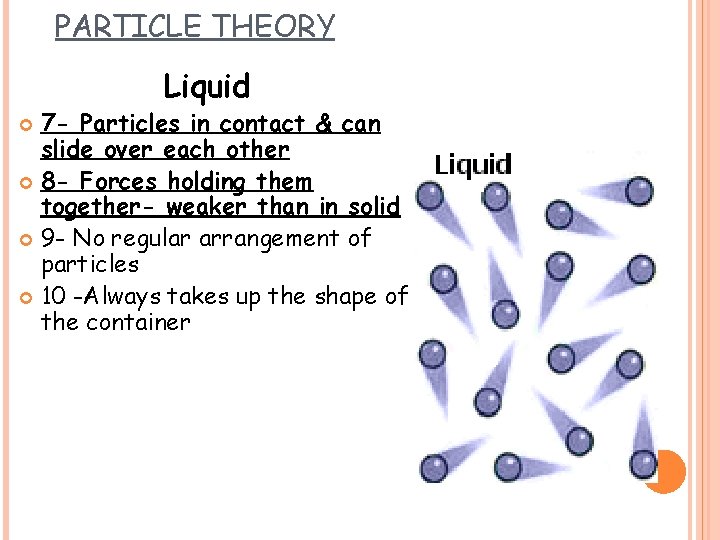
PARTICLE THEORY Liquid 7 - Particles in contact & can slide over each other 8 - Forces holding them together- weaker than in solid 9 - No regular arrangement of particles 10 -Always takes up the shape of the container

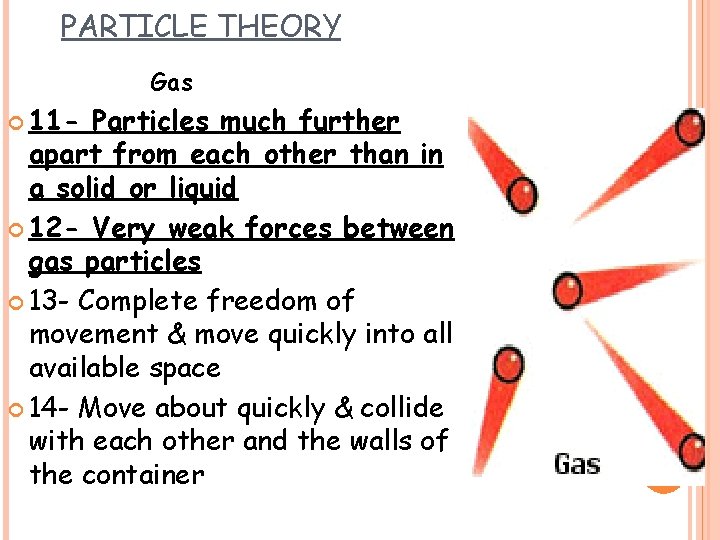
PARTICLE THEORY Gas 11 - Particles much further apart from each other than in a solid or liquid 12 - Very weak forces between gas particles 13 - Complete freedom of movement & move quickly into all available space 14 - Move about quickly & collide with each other and the walls of the container

NON-CHARACTERISTIC PROPERTIES A non-characteristic property is a physical or chemical property that is not unique to one particular substance. It could be used to describe many substances

NON-CHARACTERISTIC PROPERTIES EXAMPLES Temperature, Mass, Shape, Colour, Volume, Acidity and alkalinity (PH)

CHARACTERISTIC PROPERTIES A characteristic property is a physical or chemical property that is unique to a particular substance. It can be used to identify a substance.

CHARACTERISTIC PROPERTIES EXAMPLES Density: The amount of matter in an object, which is calculated by divided the mass by the volume D=M V Measured in g/cm 3 , g/m. L Or for larger objects: kg/m 3 , kg/L

Magnetism: The force of attraction between a magnet and a magnetic object

CHARACTERISTIC PROPERTIES EXAMPLES (CONT. ) Solubility: A measure of how well a substance can dissolve in another substance. The Solute: the substance that is dissolved The Solvent: the substance that dissolves the solute The Solution: the result of mixing a solute and a solvent

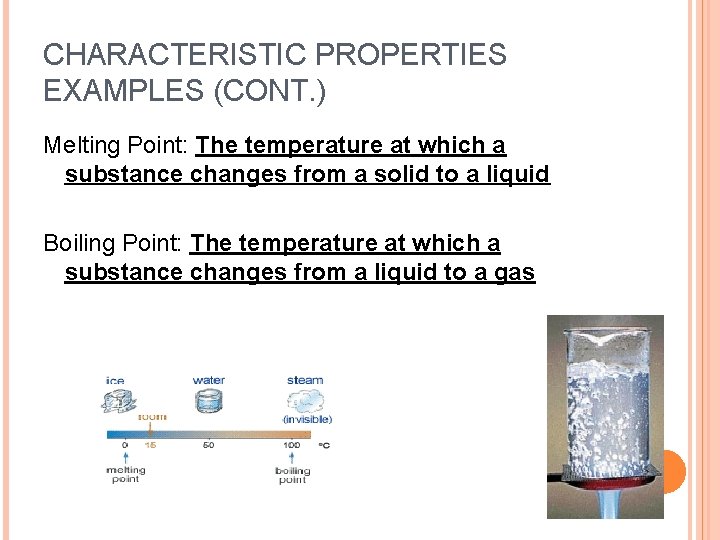
CHARACTERISTIC PROPERTIES EXAMPLES (CONT. ) Melting Point: The temperature at which a substance changes from a solid to a liquid Boiling Point: The temperature at which a substance changes from a liquid to a gas

EXAMPLE: THE ENGLISH OAK Characteristic Property Density = 720 kg/m 3 Non-Characteristic Property Light yellow to Medium brown in colour Non-Characteristic Property 25 -30 m tall

Below is the properties of unknown substances. Which of the following is a characteristic property? 1. Mass of 346 grams 2. Red and circular in shape 3. Temperature of 250 C 4. Boiling Point of 204 o. C

Identify all of the non-charactertistic properties listed below 1. Volume of 200 ml 2. Square shaped 3. Temperature of 450 C 4. Freezing Point of 204 o. C 5. Density of 24 g/ml

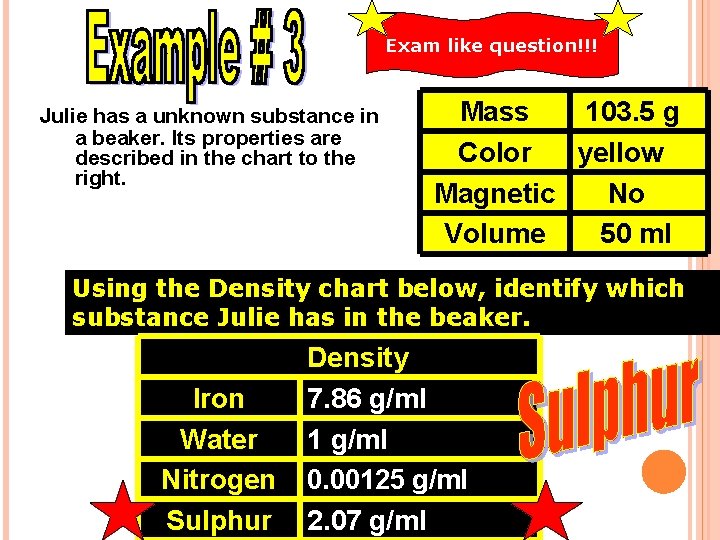
Exam like question!!! Julie has a unknown substance in a beaker. Its properties are described in the chart to the right. Mass 103. 5 g Color yellow Magnetic No Volume 50 ml Using the Density chart below, identify which substance Julie has in the beaker. Iron Water Nitrogen Sulphur Density 7. 86 g/ml 1 g/ml 0. 00125 g/ml 2. 07 g/ml

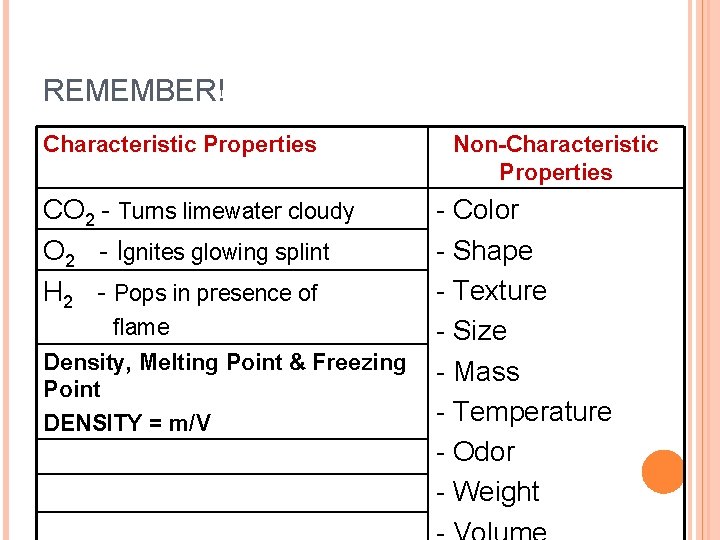
REMEMBER! Characteristic Properties CO 2 - Turns limewater cloudy O 2 - Ignites glowing splint H 2 - Pops in presence of flame Density, Melting Point & Freezing Point DENSITY = m/V Non-Characteristic Properties - Color - Shape - Texture - Size - Mass - Temperature - Odor - Weight