STATES OF MATTER Chapter 10 KINETIC MOLECULAR THEORY







- Slides: 12

STATES OF MATTER Chapter 10

KINETIC MOLECULAR THEORY (KMT) Based on the idea that particles of matter are always in motion. Ideal gas is a hypothetical gas that perfectly fits all the assumptions of the KMT

The KMT is based off 5 assumptions: 1. Gases consist of many tiny particles that are far apart. 2. Collisions between gas particles, between particles, and container walls are elastic collisions (no net loss of total kinetic energy) 3. Gas particles are in continuous , rapid, random motion. 4. No forces of attraction between gas particles 5. The temperature of a gas depends on the average kinetic energy of the particles of gas. KMT ASSUMPTIONS

No definite shape or volume Fluidity Low density Can be compressed Diffusion Effusion (gas particles pass through a tiny opening) PROPERTIES OF GASES

Definite volume No definite shape Denser than gas Less compressibility than gases Ability to diffuse Surface tension Capillary action Vaporization, Evaporation, Boiling, Freezing PROPERTIES OF LIQUIDS

Two types of solids Crystalline – nice crystal arrangement pattern Defined melting points Amorphous – particles are arranged randomly No definite melting point Definite shape and volume High density Incompressible Low rate of diffusion PROPERTIES OF SOLIDS

PHASE CHANGES Most substances can exist in three states depending on the temperature and pressure. States of substances are referred to as phases when they coexist as physically distinct parts of a mixture. Ice water is a heterogenous mixture with two phases, solid ice and liquid water. When energy is added or removed from a system, one phase can change into another. o Energy added or absorbed: Endothermic. o Energy removed or released: Exothermic. Heat is the transfer of energy from an object at a higher temperature to an object at a lower temperature. Melting point is the temperature at which a substance becomes a liquid.

EVAPORATION VS VAPORIZATION Vaporization is the process by which a liquid changes to a gas or a vapor. It requires the input of energy with molecules escaping from the surface of the liquid (heat has to be added). Evaporation is the process by which a liquid changes to a gas or vapor, at the surface of the liquid (does not require direct contact of heat). The pressure exerted by a vapor over a liquid is called vapor pressure. The boiling point of a liquid is the temperature at which the vapor pressure of a liquid equals the atmospheric pressure. Freezing point is the temperature at which a liquid is converted to a solid.

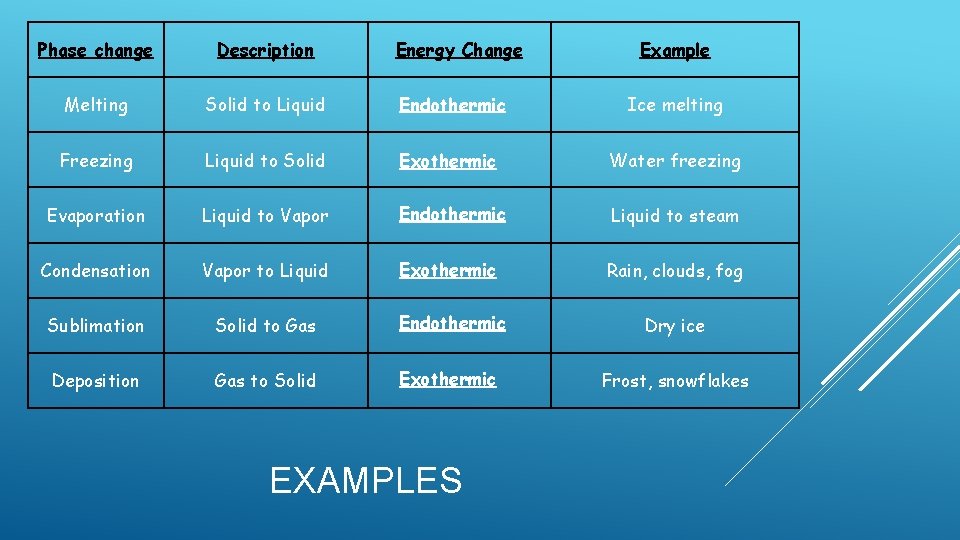
Phase change Description Energy Change Melting Solid to Liquid Endothermic Ice melting Freezing Liquid to Solid Exothermic Water freezing Evaporation Liquid to Vapor Endothermic Liquid to steam Condensation Vapor to Liquid Exothermic Rain, clouds, fog Sublimation Solid to Gas Endothermic Dry ice Deposition Gas to Solid Exothermic Frost, snowflakes EXAMPLES Example

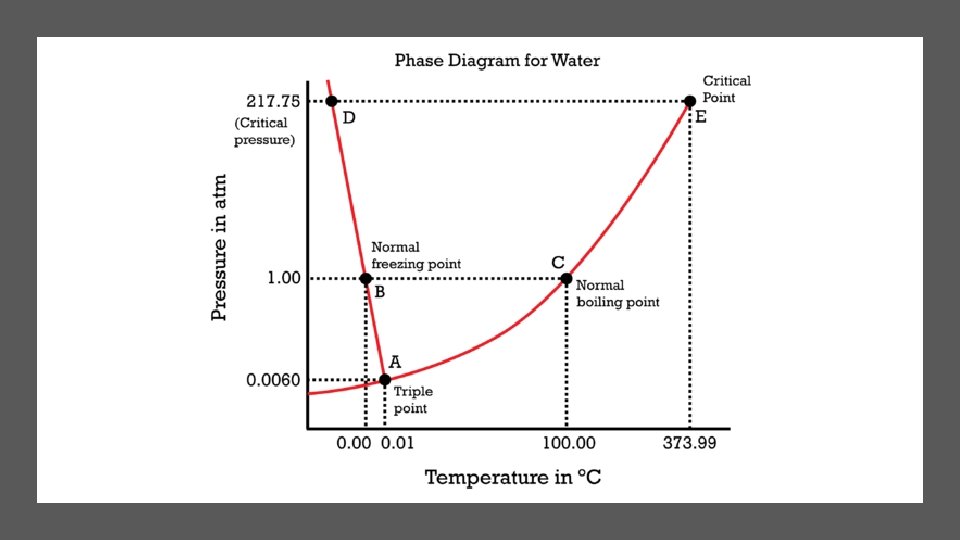
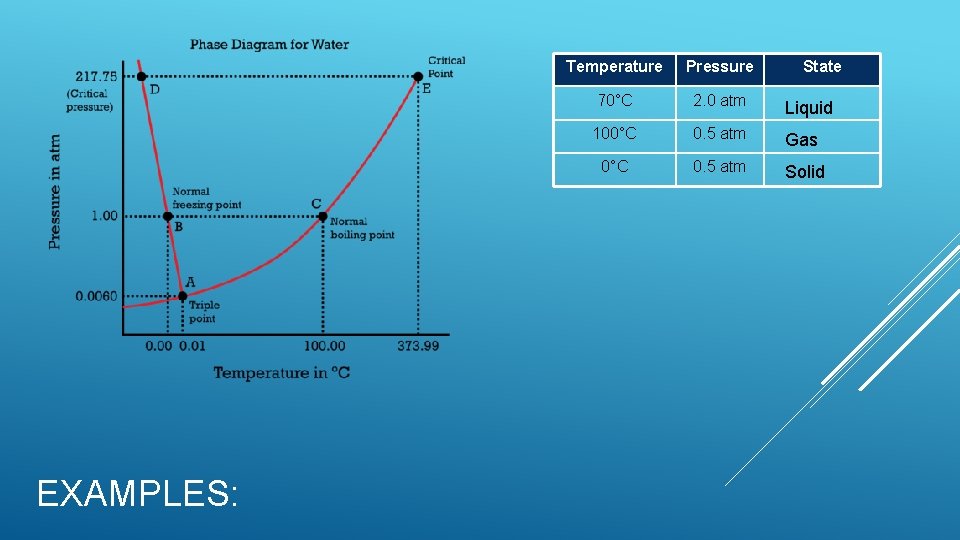
PHASE DIAGRAMS There are two variables that combine to control the phase of a substance: temperature and pressure. These two variables can have opposite effects on a substance. A phase diagram is a graph of pressure versus temperature that shows the phase of a substance under different conditions of temperature and pressure. The triple point is the point on a phase diagram that represents the temperature and pressure at which three phases of a substance coexist. Subsequently all six phase changes can occur at the triple point for water. This is indicated at point A in the diagram below. The critical point is the critical pressure and temperature above which water cannot exist as a liquid. This is indicated at point E in the diagram below. The point at which a substances freezes or boils is called the substance’s normal freezing and boiling points. For water the normal freezing point is 0 o. C and normal boiling point is 100 o. C at 1 atmosphere of pressure (standard pressure).


EXAMPLES: Temperature Pressure State 70°C 2. 0 atm Liquid 100°C 0. 5 atm Gas 0°C 0. 5 atm Solid