States of Matter Anything that has mass and





- Slides: 14

States of “Matter” Anything that has mass and takes up space (volume)

5 Physical States of Matter • Bose-Einstein (Newest State) • Solid • Liquid • Gas • Plasma

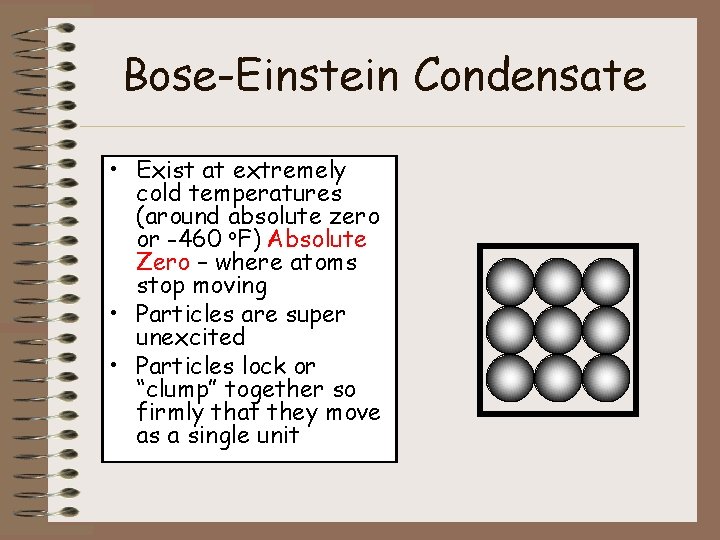
Bose-Einstein Condensate • Exist at extremely cold temperatures (around absolute zero or -460 o. F) Absolute Zero – where atoms stop moving • Particles are super unexcited • Particles lock or “clump” together so firmly that they move as a single unit

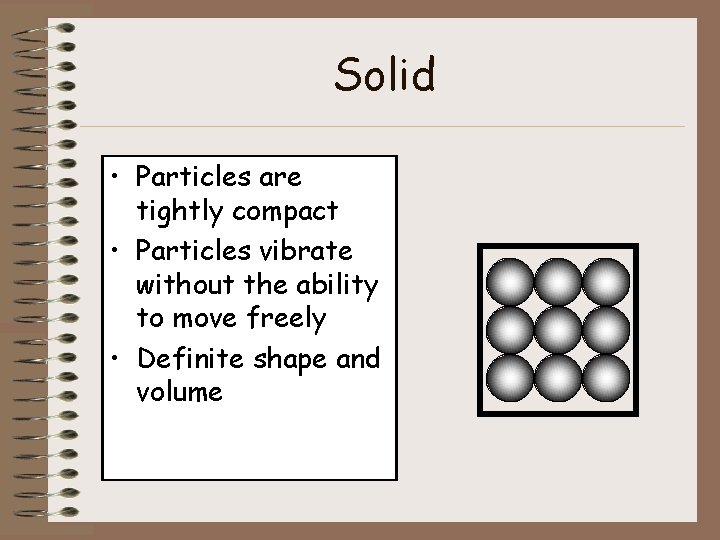
Solid • Particles are tightly compact • Particles vibrate without the ability to move freely • Definite shape and volume

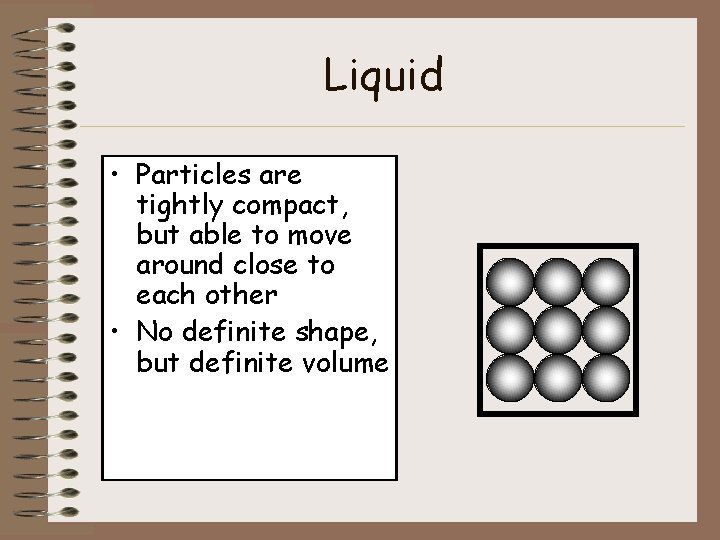
Liquid • Particles are tightly compact, but able to move around close to each other • No definite shape, but definite volume

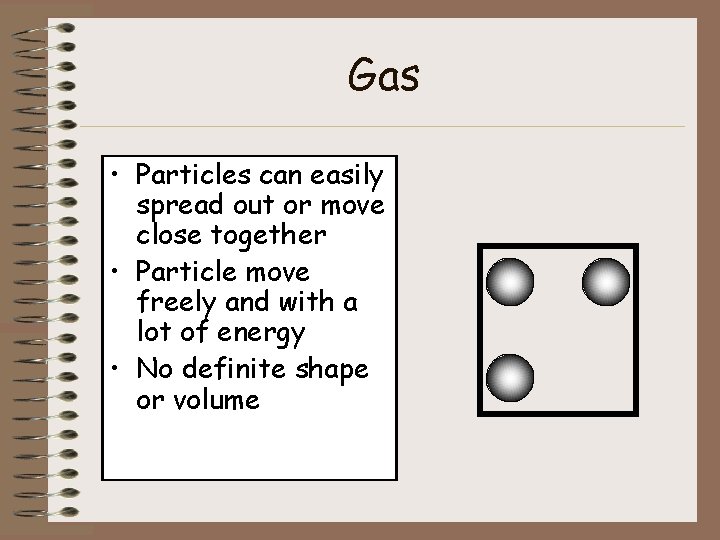
Gas • Particles can easily spread out or move close together • Particle move freely and with a lot of energy • No definite shape or volume

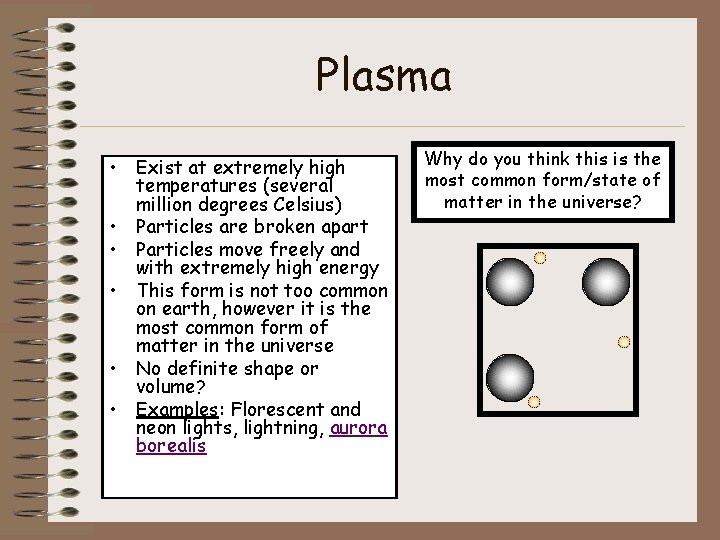
Plasma • Exist at extremely high temperatures (several million degrees Celsius) • Particles are broken apart • Particles move freely and with extremely high energy • This form is not too common on earth, however it is the most common form of matter in the universe • No definite shape or volume? • Examples: Florescent and neon lights, lightning, aurora borealis Why do you think this is the most common form/state of matter in the universe?

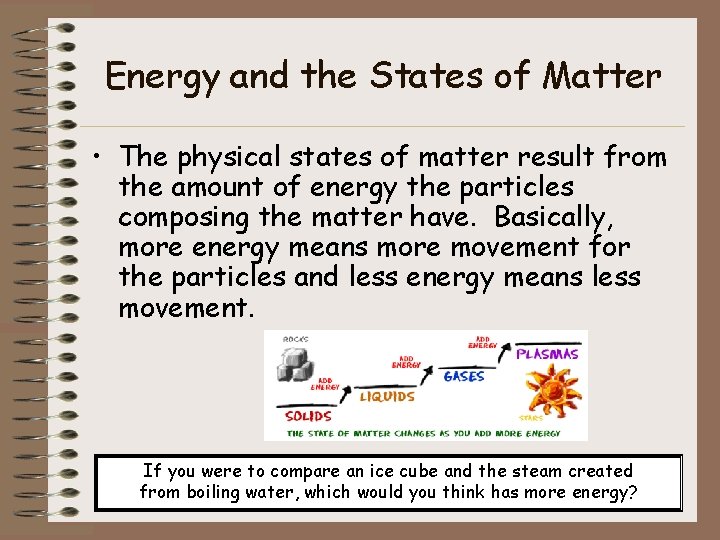
Energy and the States of Matter • The physical states of matter result from the amount of energy the particles composing the matter have. Basically, more energy means more movement for the particles and less energy means less movement. If you were to compare an ice cube and the steam created from boiling water, which would you think has more energy?

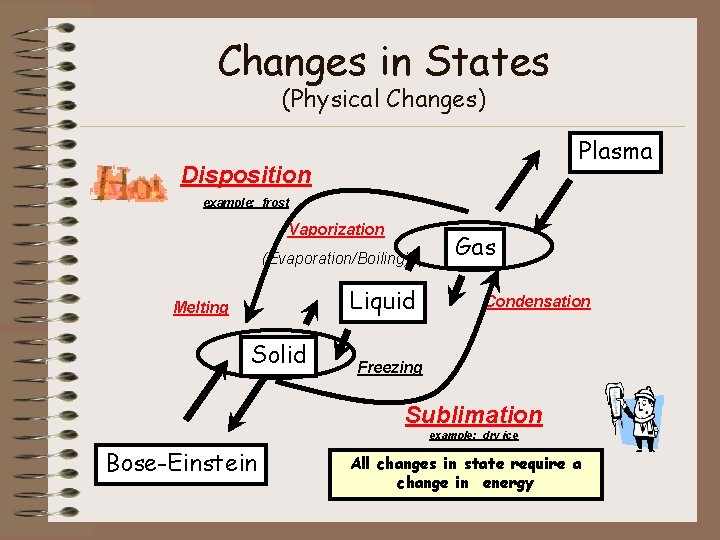
Changes in States (Physical Changes) Plasma Disposition example: frost Vaporization (Evaporation/Boiling) Liquid Melting Solid Gas Condensation Freezing Sublimation example: dry ice Bose-Einstein All changes in state require a change in energy

States of Matter Simulation 1 Simulation 2 This is what happens when energy is added and taken away

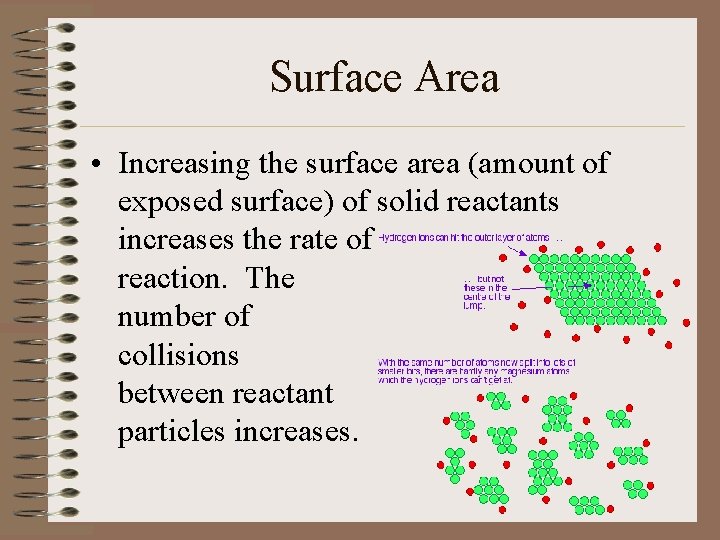
Surface Area • Increasing the surface area (amount of exposed surface) of solid reactants increases the rate of reaction. The number of collisions between reactant particles increases.

Other Examples of Surface Area Medicines get into your system faster when broken apart Water evaporates faster with exposure to air

Catalyst • A catalyst is a substance that speeds up a reaction without being permanently changed. It decreases the needed activation energy. • Example: https: //www. youtube. com/watch? v=2 qlb 8 X_ ff. O 8

Inhibitor • An inhibitor slows down or stops a reaction. Example: a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy. or