States of Matter 1 Solid has a definite

- Slides: 5

States of Matter 1. Solid = has a definite shape and volume. 2. Liquid = has definite volume but no definite shape. Its particles can flow freely. 3. Gas = has no definite shape and volume. It is highly compressible. 4. Plasma = is an ionized gas of electrons and protons that exists only at very high temperature such as those in the stars and atomic bomb. It was identified by Sir William Crookes in 1879. 5. Bose-Einstein Condensate (BEC) = is a condensate which is composed of atoms chilled to less than a millionth of a degree above the absolute zero. These atoms have lost their individual identities. Satyendra Nath Bose in 1920’s studied it and then Einstein made a theory to strengthen Bose’s idea.

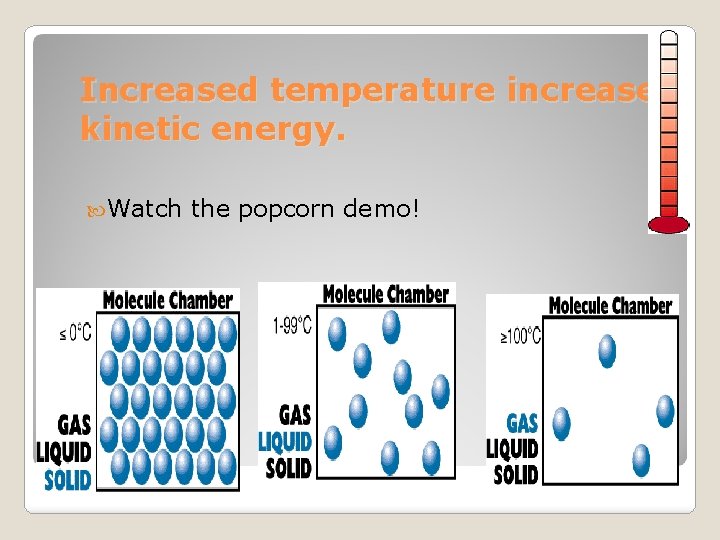
Increased temperature increases kinetic energy. Watch the popcorn demo!

Drill: (Copy) Properties of Matter Physical Properties Can be observed or measured by the senses Extensivedepends on the amount of the material Intensive – depends on the kind of the material Ex. mass, length, volume, size, Ex. density, temperature, melting point, color, taste Chemical Properties can only be observed or measured after the material reacts with another material Ex. Ability to rust, flammability

Changes of Matter Physical – changes the physical properties of matter; no new substance; the material remains the same Examples: all phase changes, tearing, breaking Chemical – changes the composition/chemi cal properties of matter; new substance is formed Examples: burning, rusting, digesting, growing

Energy Changes endothermic – absorbing heat Examples: Bond breaking such as from solid to liquid (BENDO) Exothermic – giving off heat Examples: Bond Forming such as liquid to solid (FEXO)