State of the Science on Sources of Carbonaceous

State of the Science on Sources of Carbonaceous Aerosols and Their Contribution to Regional Haze John G. Watson (johnw@dri. edu) Judith C. Chow Desert Research Institute, Reno, NV, USA Presented at: WRAP Workshop on Fire, Carbon, and Dust May 23 -24, 2006 Sacramento, CA

Objectives • Describe sources of carbonaceous aerosol • Identify and evaluate methods to identify and quantify carbon emissions and source contributions • Review progress on reconciling different carbon measurement methods and instruments
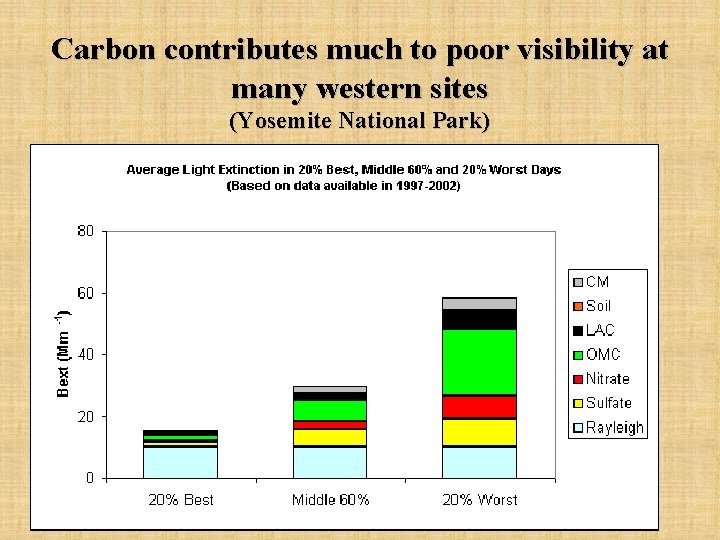
Carbon contributes much to poor visibility at many western sites (Yosemite National Park)
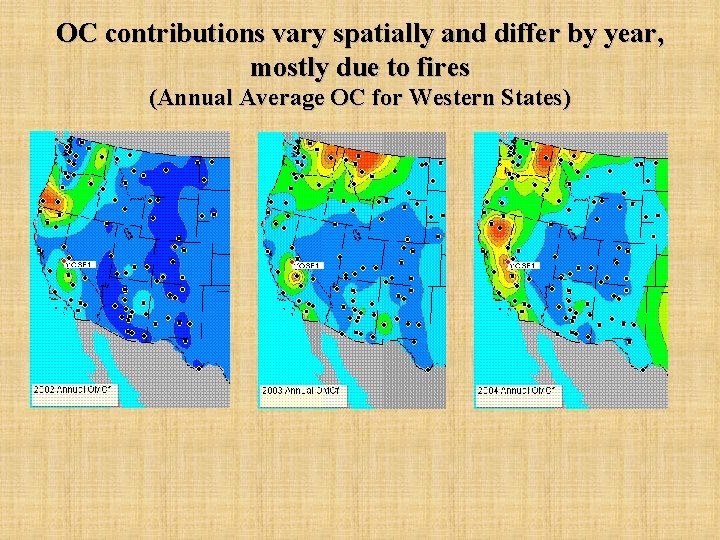
OC contributions vary spatially and differ by year, mostly due to fires (Annual Average OC for Western States)

Fire contributions vary with time during the year (Yosemite National Park)
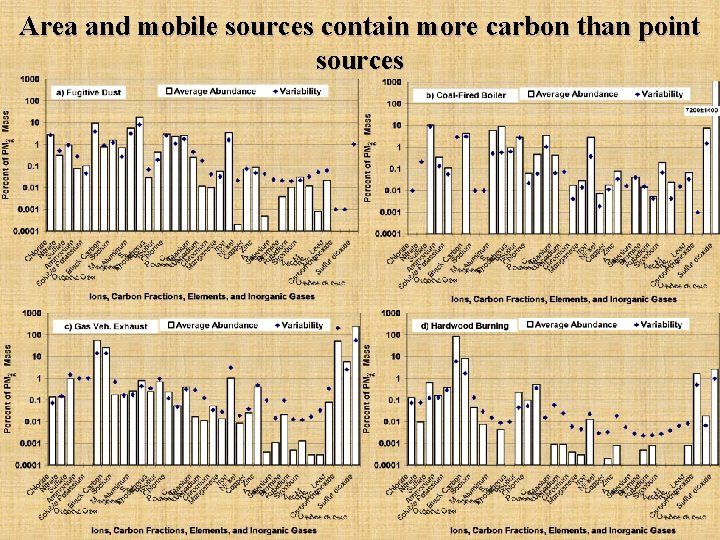
Area and mobile sources contain more carbon than point sources

Emissions relevant to carbonaceous PM • PM Fugitive dust from wind erosion, agricultural activities, construction, storage piles, and vehicle traffic on paved and unpaved roads. • VOC Vegetation, surface coatings, fuel storage and distribution, solvents. • PM, VOC Burning and cooking from stoves, charbroilers, trash, forest fires, and agricultural burning. • PM, NOx, VOC Ducted exhaust from industrial facilities (e. g. , coal- and oil-fired power stations, smelting, cement plants, chemical plants, petroleum extraction and refining, glass manufacturing, paper making, shipping). Vehicle exhaust from cars, trucks, motorcycles, and buses. Exhaust from non-road generators, small engines, non-road vehicles.

Potassium is a reaonsable indicator of fire contributions, but there’s still noise
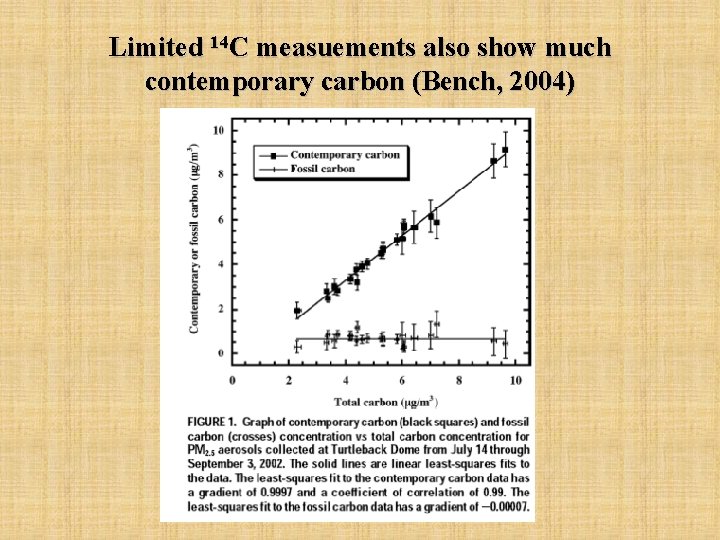
Limited 14 C measuements also show much contemporary carbon (Bench, 2004)
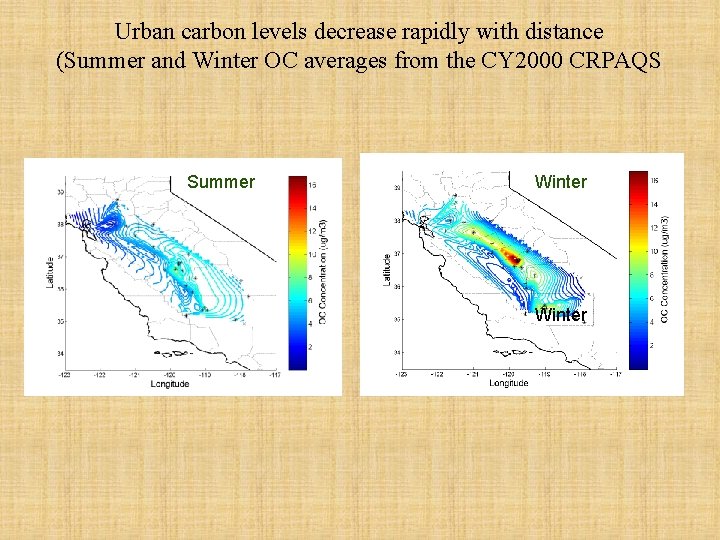
Urban carbon levels decrease rapidly with distance (Summer and Winter OC averages from the CY 2000 CRPAQS Nitrate at Fresno Summer Winter
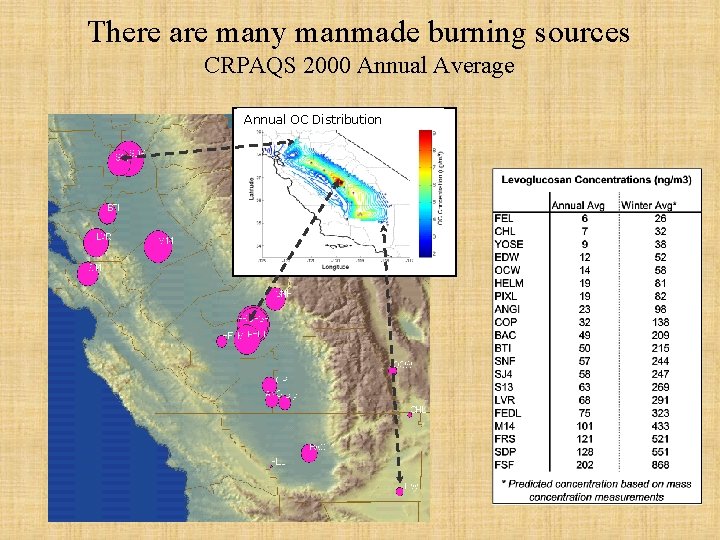
There are many manmade burning sources CRPAQS 2000 Annual Average Annual OC Distribution Summer Winter

Issues for PM Carbon Emission Rates and Compositions • Many carbon compounds are semi-volatile and condense or evaporate depending on vapor pressure, temperature, and surface availablility on other particles • Different (certification) test methods for different source types result in different emission rates and compositions for the same equipment, fuels, and operating conditions • Organic vapors adsorb onto quartz fiber filters used to measure carbon • Size distributions, compositions, and gas/particle phases continue to change within and emissions inventory grid. Grid scaling affects equivalent emissions
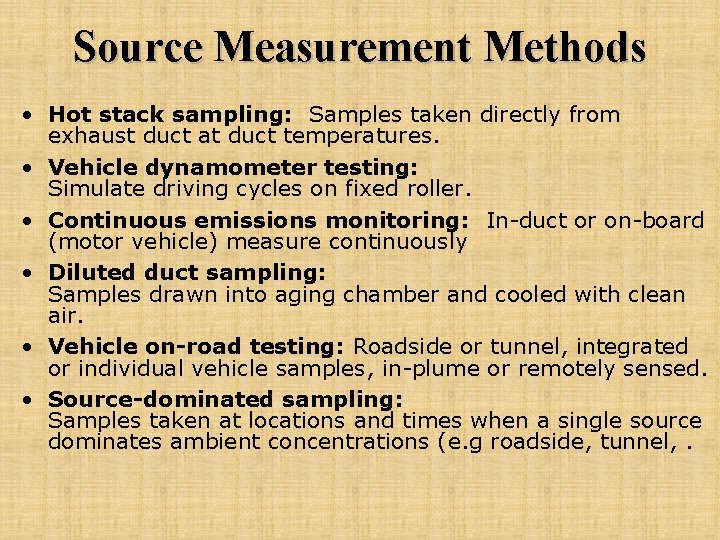
Source Measurement Methods • Hot stack sampling: Samples taken directly from exhaust duct at duct temperatures. • Vehicle dynamometer testing: Simulate driving cycles on fixed roller. • Continuous emissions monitoring: In-duct or on-board (motor vehicle) measure continuously • Diluted duct sampling: Samples drawn into aging chamber and cooled with clean air. • Vehicle on-road testing: Roadside or tunnel, integrated or individual vehicle samples, in-plume or remotely sensed. • Source-dominated sampling: Samples taken at locations and times when a single source dominates ambient concentrations (e. g roadside, tunnel, .
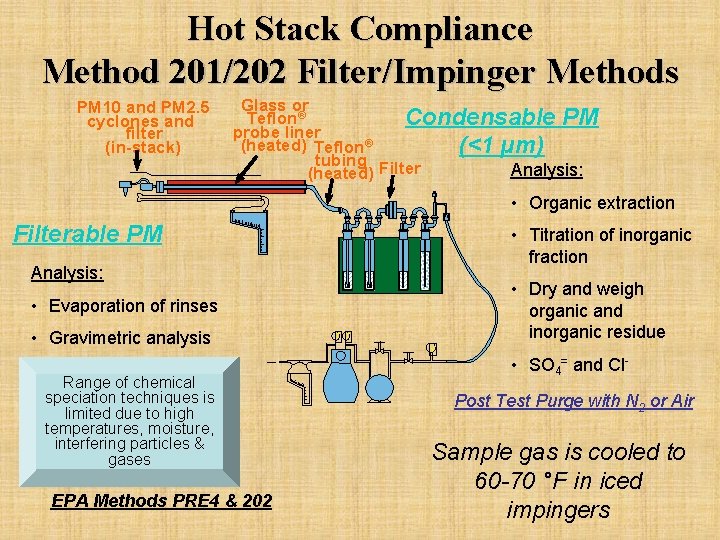
Hot Stack Compliance Method 201/202 Filter/Impinger Methods PM 10 and PM 2. 5 cyclones and filter (in-stack) Glass or Teflon® Condensable PM probe liner (heated) Teflon® (<1 µm) tubing Filter Analysis: (heated) • Organic extraction Filterable PM • Titration of inorganic fraction Analysis: • Dry and weigh organic and inorganic residue • Evaporation of rinses • Gravimetric analysis Range of chemical speciation techniques is limited due to high temperatures, moisture, interfering particles & gases EPA Methods PRE 4 & 202 T T V • SO 4= and Cl. Post Test Purge with N 2 or Air Sample gas is cooled to 60 -70 °F in iced impingers
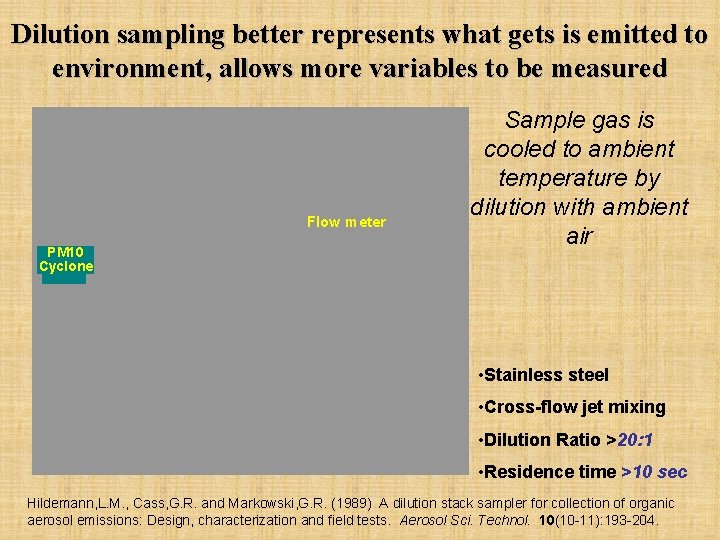
Dilution sampling better represents what gets is emitted to environment, allows more variables to be measured Flow meter PM 10 Cyclone Sample gas is cooled to ambient temperature by dilution with ambient air • Stainless steel • Cross-flow jet mixing • Dilution Ratio >20: 1 • Residence time >10 sec Hildemann, L. M. , Cass, G. R. and Markowski, G. R. (1989) A dilution stack sampler for collection of organic aerosol emissions: Design, characterization and field tests. Aerosol Sci. Technol. 10(10 -11): 193 -204.

Difference in PM 2. 5 Mass between In-Stack and Dilution Sampling Gas-Fired Boiler - Field Data 0. 018 In-Stack Methods 0. 016 0. 014 inorganic condensable (M 202) Filterable PM (M 201 A) PM 2. 5 (dilution) lb/MMBtu 0. 012 0. 01 0. 008 0. 006 0. 004 Dilution Method 0. 002 0 Run 1 Run 2 Run 3 AP 42 Chang, M. C. and England, G. C. (2004) Development of fine particulate emission factors and speciation profiles for oil and gas-fired combustion systems, Update: Critical review of source sampling and analysis methodologies for characterizing organic aerosol and fine particulate source emission profiles. Irvine, CA: GE Energy and Environmental Research Corp.

Mobile source certification requires dilution Dilution tunnel and sampling ports Put generator on wheels and move it and it is certified by dilution sampling Install the generator permanently and it is certified by hot stack sampling and has different emissions
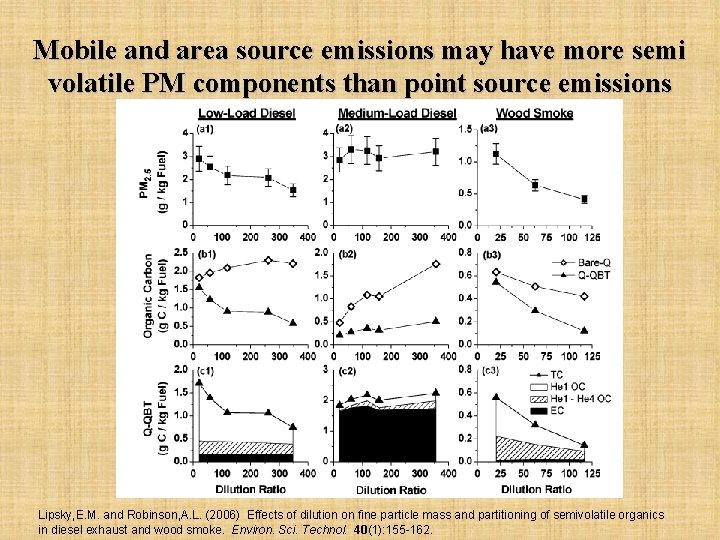
Mobile and area source emissions may have more semi volatile PM components than point source emissions Lipsky, E. M. and Robinson, A. L. (2006) Effects of dilution on fine particle mass and partitioning of semivolatile organics in diesel exhaust and wood smoke. Environ. Sci. Technol. 40(1): 155 -162.
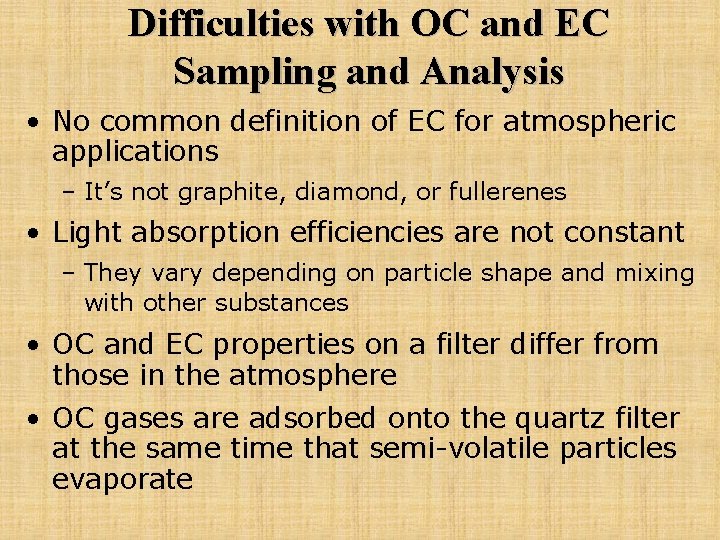
Difficulties with OC and EC Sampling and Analysis • No common definition of EC for atmospheric applications – It’s not graphite, diamond, or fullerenes • Light absorption efficiencies are not constant – They vary depending on particle shape and mixing with other substances • OC and EC properties on a filter differ from those in the atmosphere • OC gases are adsorbed onto the quartz filter at the same time that semi-volatile particles evaporate
Thermal Evolution Methods are Conceptually Simple Lavoisier's Oil Analysis "Traité Élémentaire de Chimie" (1789) vol. II, chap. VII, p. 493 -501
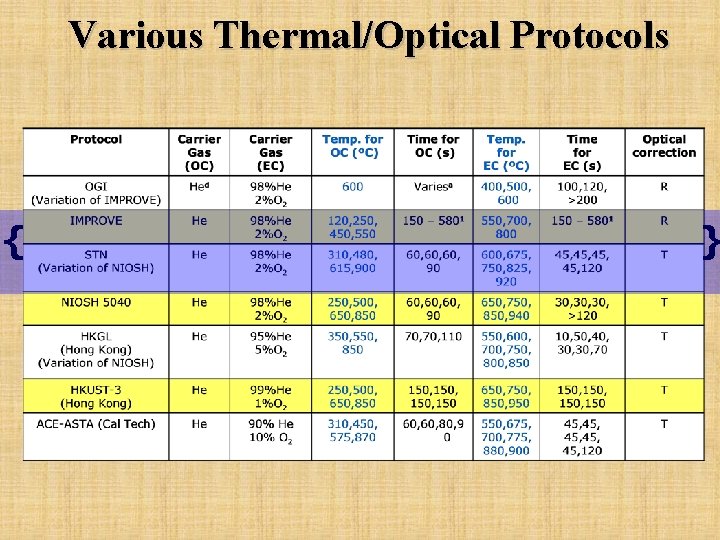
Various Thermal/Optical Protocols { }
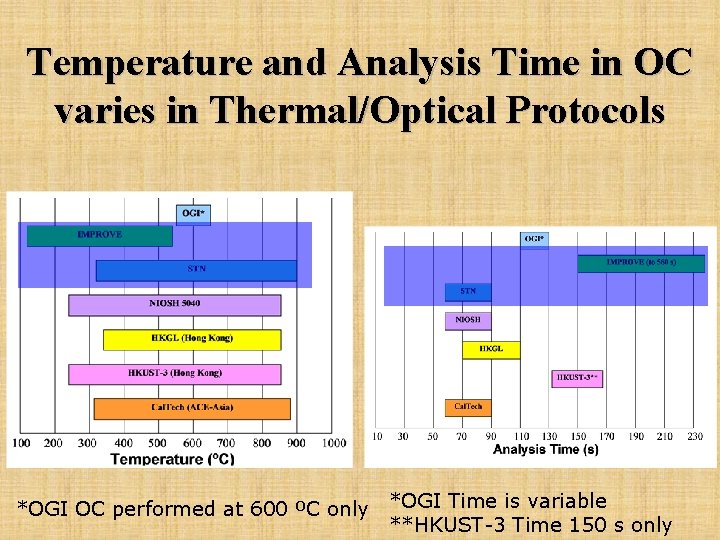
Temperature and Analysis Time in OC varies in Thermal/Optical Protocols *OGI OC performed at 600 ºC only *OGI Time is variable **HKUST-3 Time 150 s only
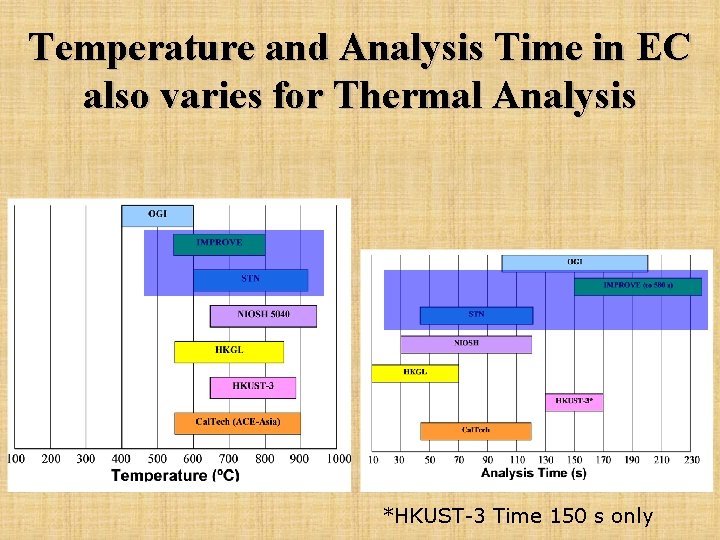
Temperature and Analysis Time in EC also varies for Thermal Analysis *HKUST-3 Time 150 s only
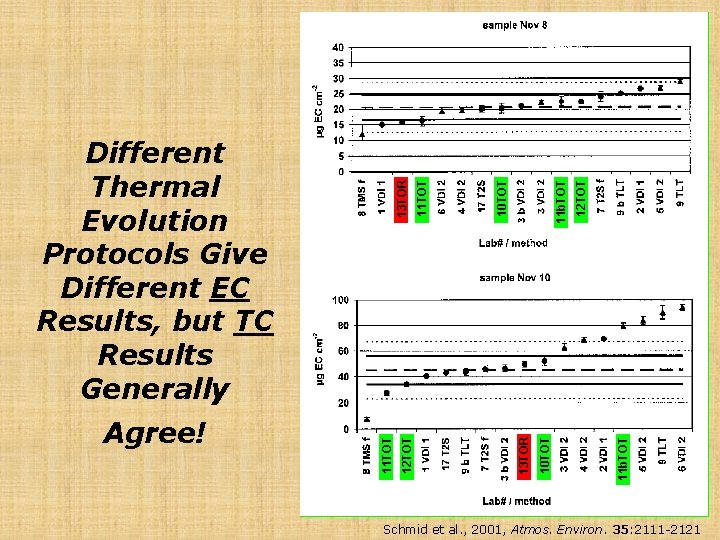
11 b. TOT 12 TOT 11 b. TOT 10 TOT 11 TOT 10 TOT 13 TOR 12 TOT 11 TOT Agree! 13 TOR Different Thermal Evolution Protocols Give Different EC Results, but TC Results Generally Schmid et al. , 2001, Atmos. Environ. 35: 2111 -2121

Learned much in transition from old to new IMPROVE analyzers DRI/OGC Analyzer DRI Model 2001 Analyzer
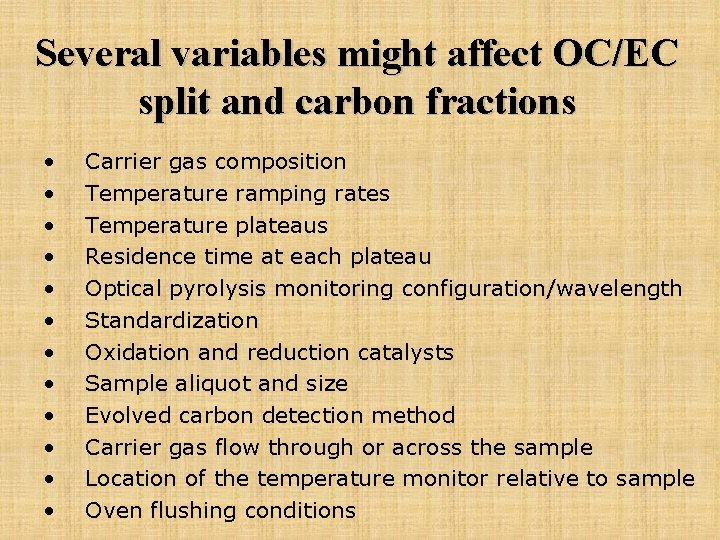
Several variables might affect OC/EC split and carbon fractions • • • Carrier gas composition Temperature ramping rates Temperature plateaus Residence time at each plateau Optical pyrolysis monitoring configuration/wavelength Standardization Oxidation and reduction catalysts Sample aliquot and size Evolved carbon detection method Carrier gas flow through or across the sample Location of the temperature monitor relative to sample Oven flushing conditions
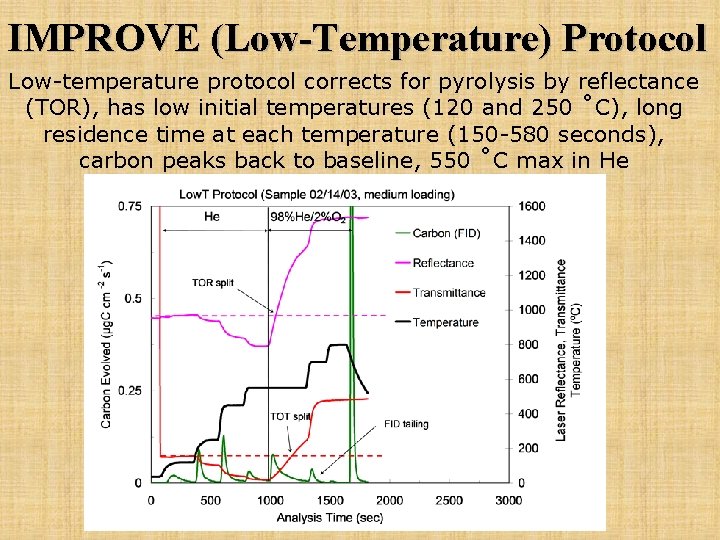
IMPROVE (Low-Temperature) Protocol Low-temperature protocol corrects for pyrolysis by reflectance (TOR), has low initial temperatures (120 and 250 ˚C), long residence time at each temperature (150 -580 seconds), carbon peaks back to baseline, 550 ˚C max in He
STN (High-Temperature) Protocol High-temperature protocol corrects for pyrolysis by transmittance (TOT), has high initial temperature (310 ˚C), fixed and short residence times (45 -120 seconds), 900 ˚C max in He
EC differs within Protocol between Reflectance (TOR) and Transmittance (TOT) Pyrolysis Corrections Chow et al. , 2001, Aerosol Sci. Technol. 34: 23 -34

IMPROVE-TOR and STN-TOR yield the Same EC. Why? Chow et al. , 2001, Aerosol Sci. Technol. 34: 23 -34
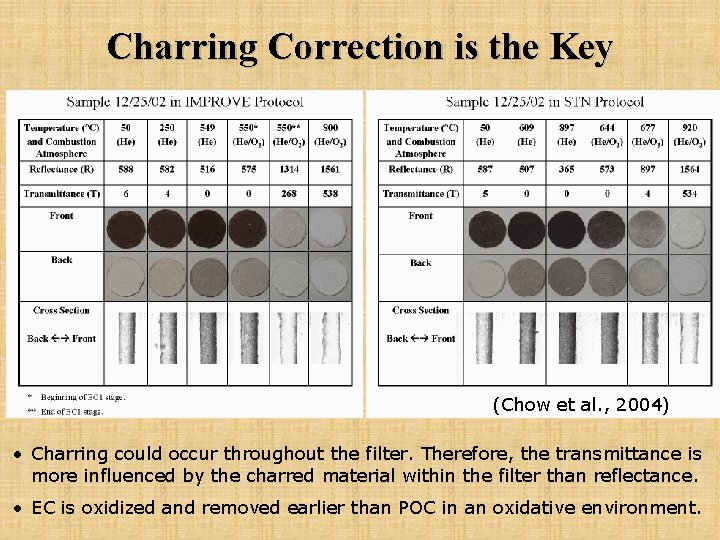
Charring Correction is the Key (Chow et al. , 2004) • Charring could occur throughout the filter. Therefore, the transmittance is more influenced by the charred material within the filter than reflectance. • EC is oxidized and removed earlier than POC in an oxidative environment.
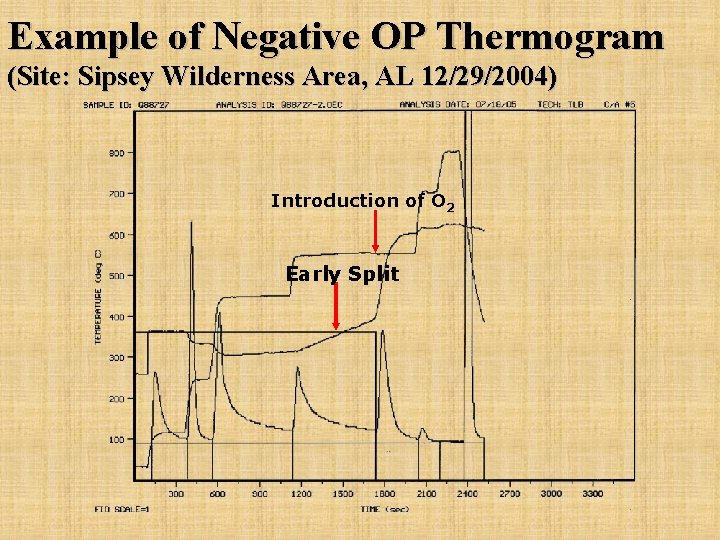
Example of Negative OP Thermogram (Site: Sipsey Wilderness Area, AL 12/29/2004) Introduction of O 2 Early Split
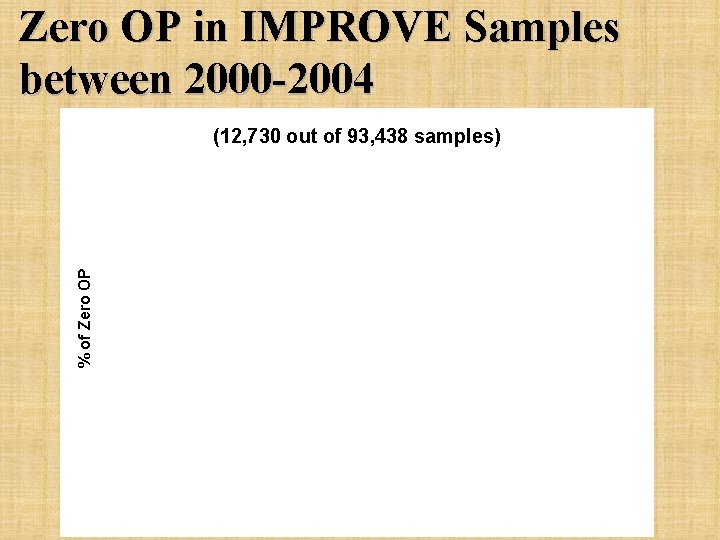
Zero OP in IMPROVE Samples between 2000 -2004 % of Zero OP (12, 730 out of 93, 438 samples)

Carbon Fractions vary by Sources
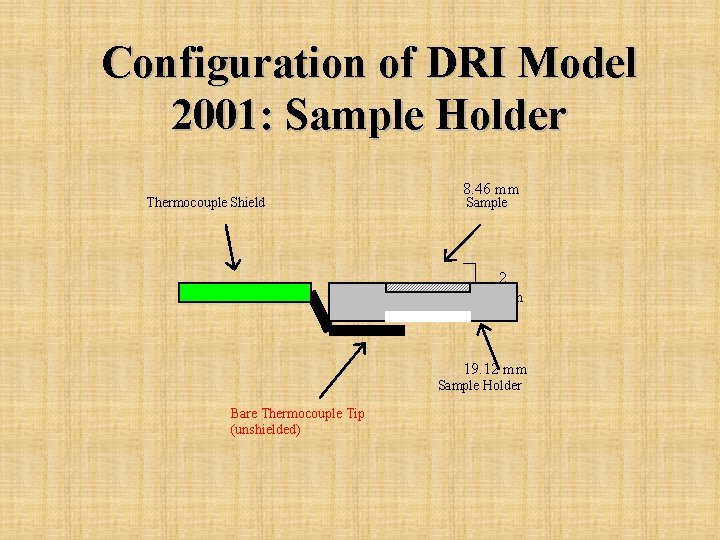
Configuration of DRI Model 2001: Sample Holder Thermocouple Shield 8. 46 mm Sample 2 mm 19. 12 mm Sample Holder Bare Thermocouple Tip (unshielded)
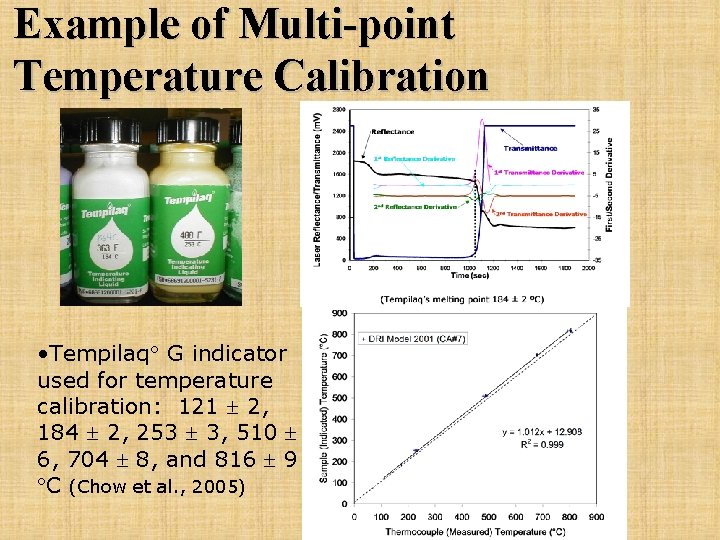
Example of Multi-point Temperature Calibration • Tempilaq G indicator used for temperature calibration: 121 2, 184 2, 253 3, 510 6, 704 8, and 816 9 C (Chow et al. , 2005)
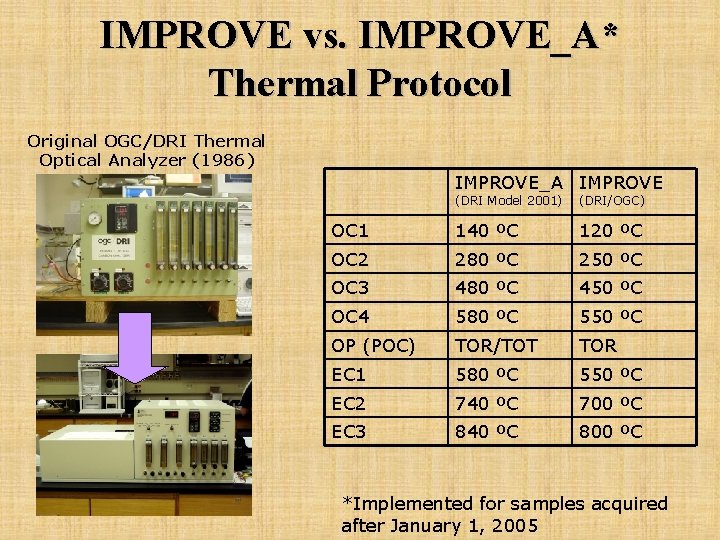
IMPROVE vs. IMPROVE_A* Thermal Protocol Original OGC/DRI Thermal Optical Analyzer (1986) IMPROVE_A IMPROVE (DRI Model 2001) (DRI/OGC) OC 1 140 ºC 120 ºC OC 2 280 ºC 250 ºC OC 3 480 ºC 450 ºC OC 4 580 ºC 550 ºC OP (POC) TOR/TOT TOR EC 1 580 ºC 550 ºC EC 2 740 ºC 700 ºC EC 3 840 ºC 800 ºC *Implemented for samples acquired after January 1, 2005
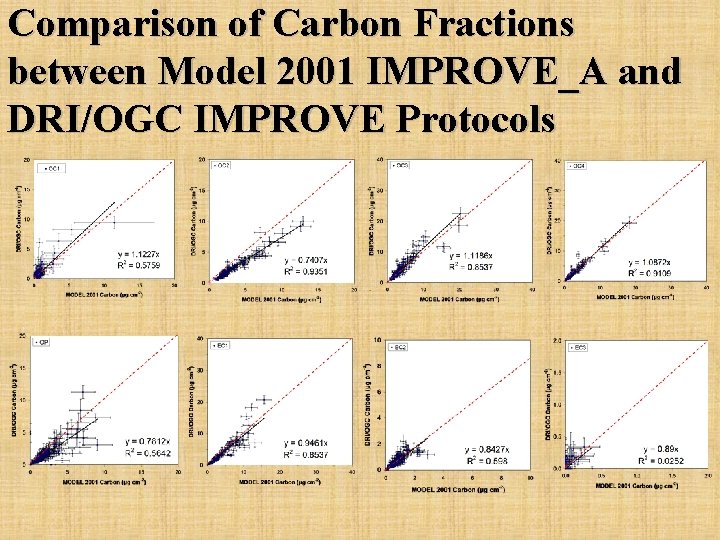
Comparison of Carbon Fractions between Model 2001 IMPROVE_A and DRI/OGC IMPROVE Protocols
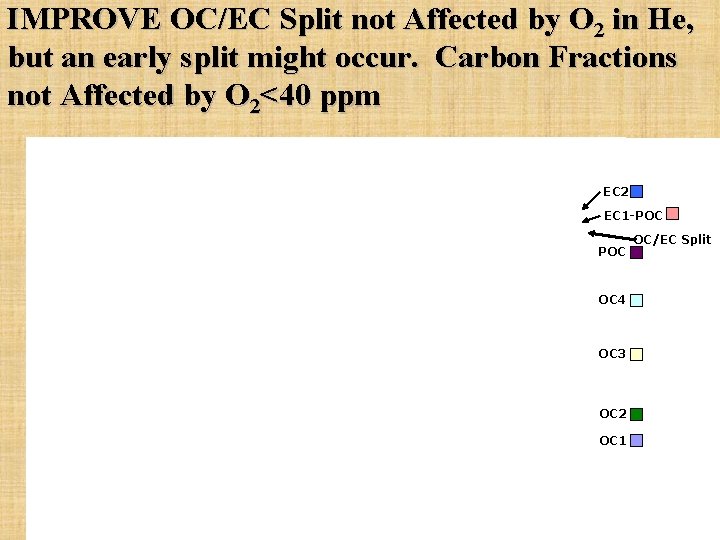
IMPROVE OC/EC Split not Affected by O 2 in He, but an early split might occur. Carbon Fractions not Affected by O 2<40 ppm EC 2 EC 1 -POC OC 4 OC 3 OC 2 OC 1 OC/EC Split
Minerals increase EC oxidation rate at higher temperatures in 100% He atmosphere. More early splits for high temperature STN
Na. Cl and other catalysts affect carbon fractions and can cause an early split
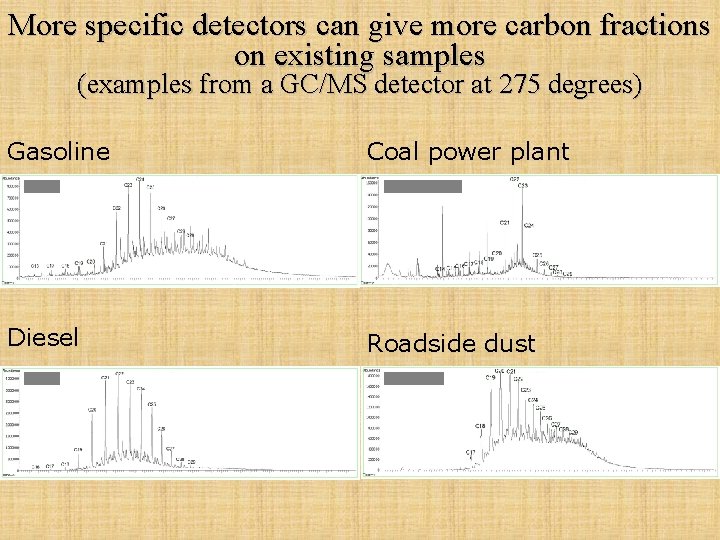
More specific detectors can give more carbon fractions on existing samples (examples from a GC/MS detector at 275 degrees) Gasoline Coal power plant Diesel Roadside dust

How can we Maximize Utility of STN and IMPROVE for Different Purposes? • Understand OC and EC – Report both TOT and TOR corrections – Report negative pyrolysis corrections – Report initial, minimum, and final reflectance and transmittance – Re-analyze fraction of samples on old analyzers • Source attribution (also needed in source samples) – Define temperature plateaus that bracket dominant compounds – Use more specific detectors
Conclusions • Very large carbon concentrations are often due to fire. These can be identified by spatial and temporal changes in carbon • Urban carbon concentrations decrease rapidly with distance • Need more specific markers for non-fire sources and for fire contributions at normal carbon levels • OC/EC split appears to be independent of temperature of program. • Oxygen in the carrier gas, catalysts such as Na. Cl, and mineral oxides affect carbon fractions and may cause a negative OC/EC pyrolysis correction • More information can be obtained from the samples with more specific carbon detectors
- Slides: 44