StartUp What is the molar mass for Calcium






- Slides: 19

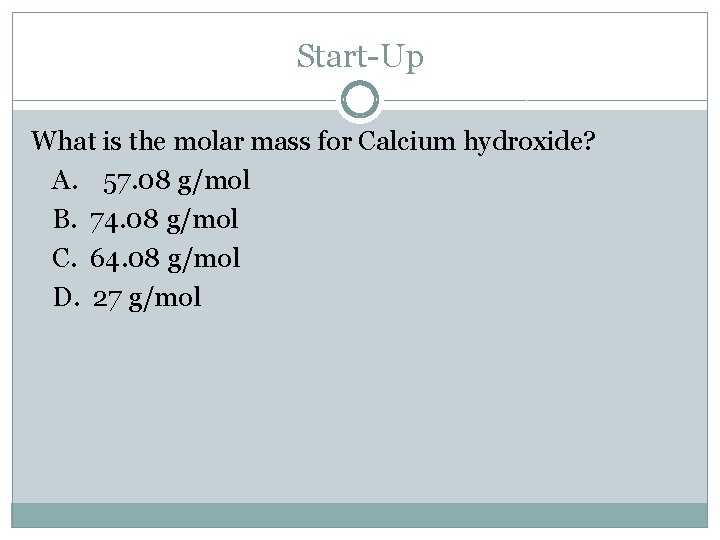
Start-Up What is the molar mass for Calcium hydroxide? A. 57. 08 g/mol B. 74. 08 g/mol C. 64. 08 g/mol D. 27 g/mol

Stoichiometry

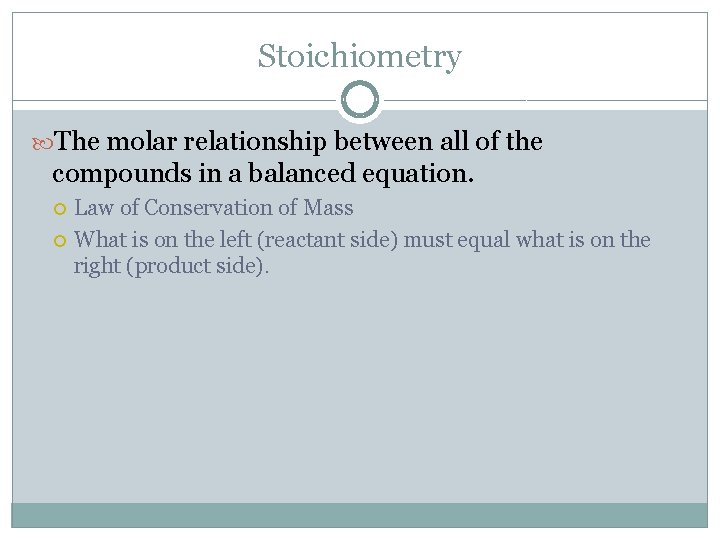
Stoichiometry The molar relationship between all of the compounds in a balanced equation. Law of Conservation of Mass What is on the left (reactant side) must equal what is on the right (product side).

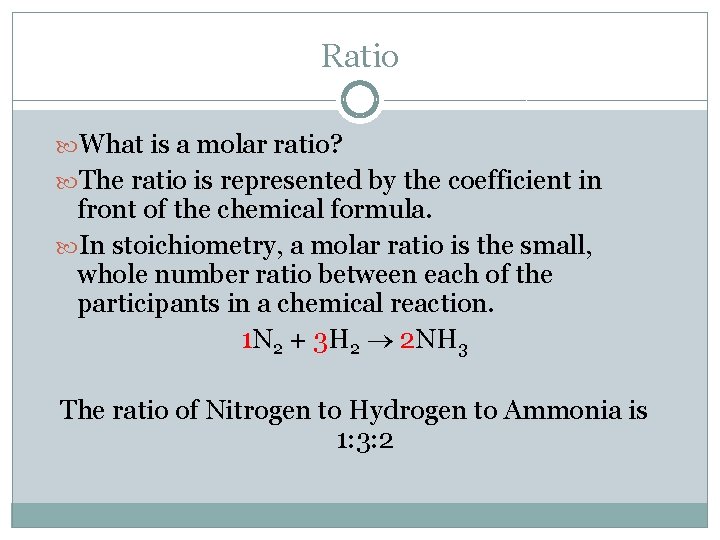
Ratio What is a molar ratio? The ratio is represented by the coefficient in front of the chemical formula. In stoichiometry, a molar ratio is the small, whole number ratio between each of the participants in a chemical reaction. 1 N 2 + 3 H 2 2 NH 3 The ratio of Nitrogen to Hydrogen to Ammonia is 1: 3: 2

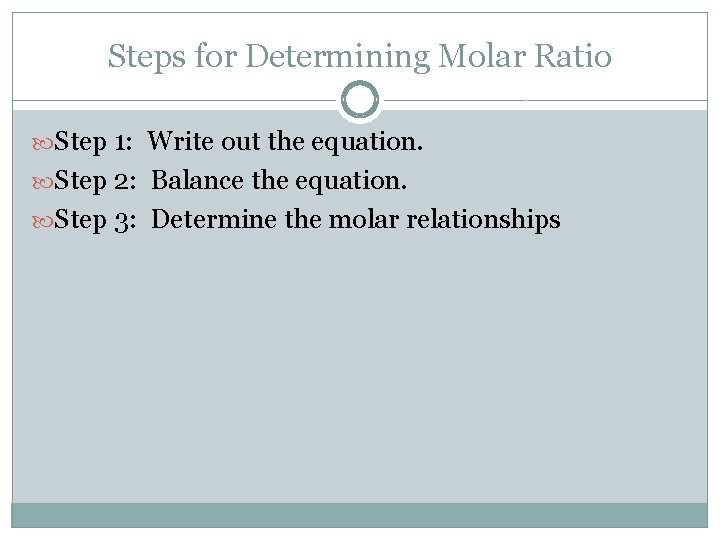
Steps for Determining Molar Ratio Step 1: Write out the equation. Step 2: Balance the equation. Step 3: Determine the molar relationships

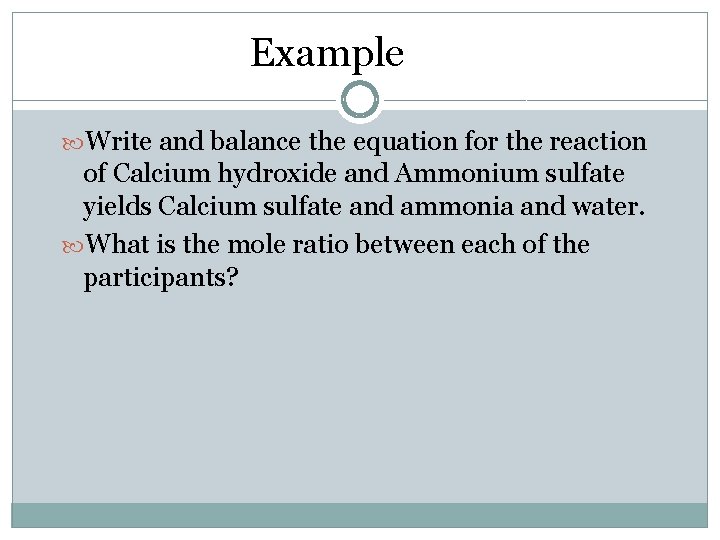
Example Write and balance the equation for the reaction of Calcium hydroxide and Ammonium sulfate yields Calcium sulfate and ammonia and water. What is the mole ratio between each of the participants?

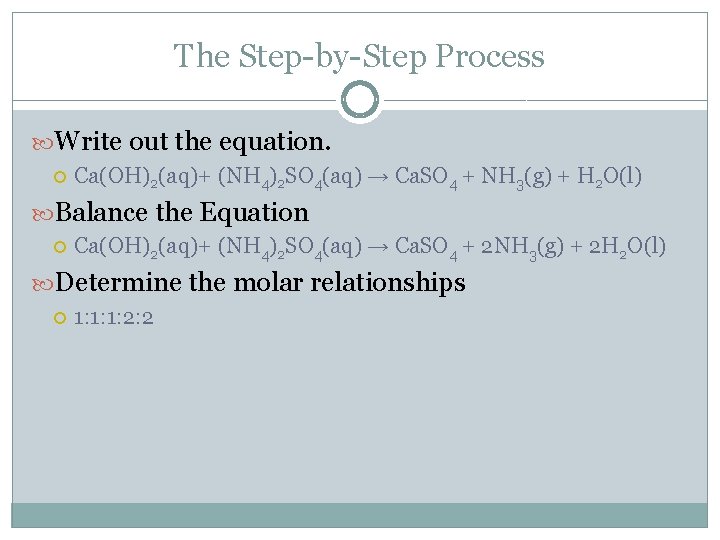
The Step-by-Step Process Write out the equation. Ca(OH)2(aq)+ (NH 4)2 SO 4(aq) → Ca. SO 4 + NH 3(g) + H 2 O(l) Balance the Equation Ca(OH)2(aq)+ (NH 4)2 SO 4(aq) → Ca. SO 4 + 2 NH 3(g) + 2 H 2 O(l) Determine the molar relationships 1: 1: 1: 2: 2

Another example Write and balance the reaction of sodium with water to yield sodium hydroxide and hydrogen gas. What are the ratios? 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) The ratio is 2: 2: 2: 1

Now try this one Write and balance the equation for the reaction of ammonia with oxygen to yield nitric acid (HNO 3) and water. NH 3(g) + 2 O 2(g) → HNO 3(aq) + H 2 O(l) The ratio is 1: 2: 1: 1

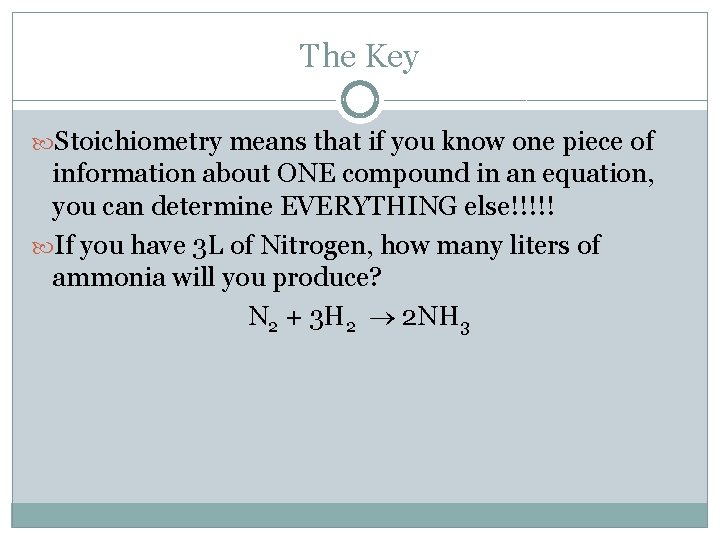
The Key Stoichiometry means that if you know one piece of information about ONE compound in an equation, you can determine EVERYTHING else!!!!! If you have 3 L of Nitrogen, how many liters of ammonia will you produce? N 2 + 3 H 2 2 NH 3

Think of it as a recipe Let’s look at that last reaction again. N 2(g) + 3 H 2(g) 2 NH 3(g) If you start out with 1 mole of Nitrogen gas and 3 moles of Hydrogen gas, you will make 2 moles of Ammonia gas. It is important in industry to know the exact proportions of your ingredients so that you will not have excess waste in your product.

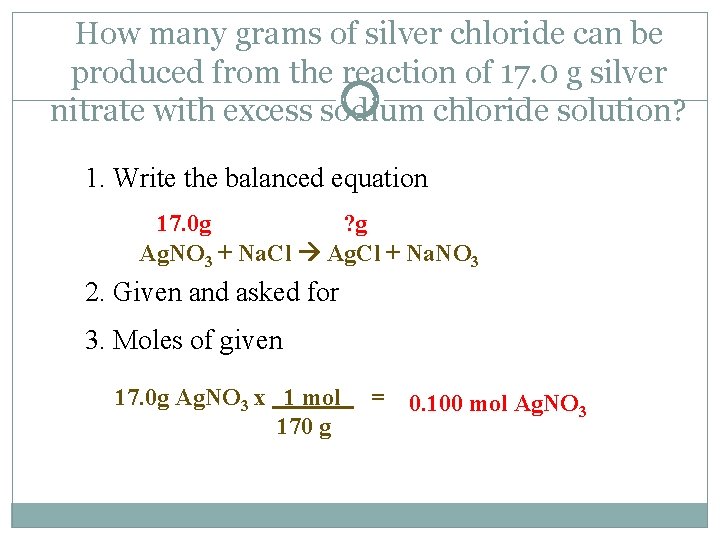
How many grams of silver chloride can be produced from the reaction of 17. 0 g silver nitrate with excess sodium chloride solution? 1. Write the balanced equation 17. 0 g ? g Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3 2. Given and asked for 3. Moles of given 17. 0 g Ag. NO 3 x 1 mol 170 g = 0. 100 mol Ag. NO 3

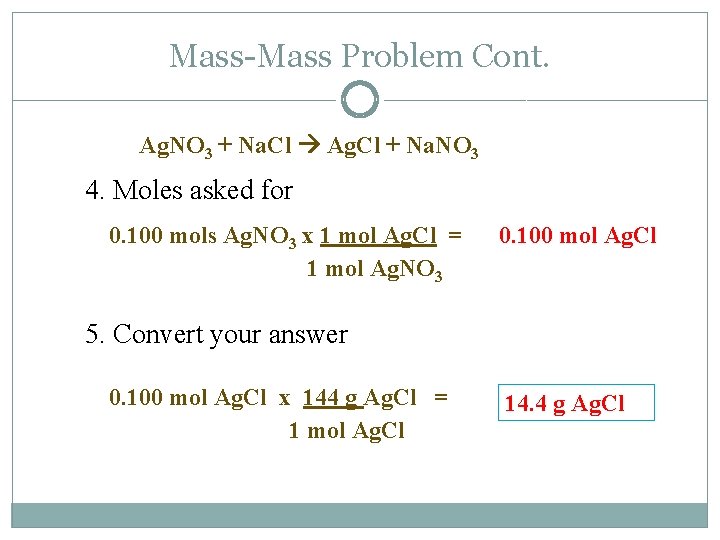
Mass-Mass Problem Cont. Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3 4. Moles asked for 0. 100 mols Ag. NO 3 x 1 mol Ag. Cl = 1 mol Ag. NO 3 0. 100 mol Ag. Cl 5. Convert your answer 0. 100 mol Ag. Cl x 144 g Ag. Cl = 1 mol Ag. Cl 14. 4 g Ag. Cl

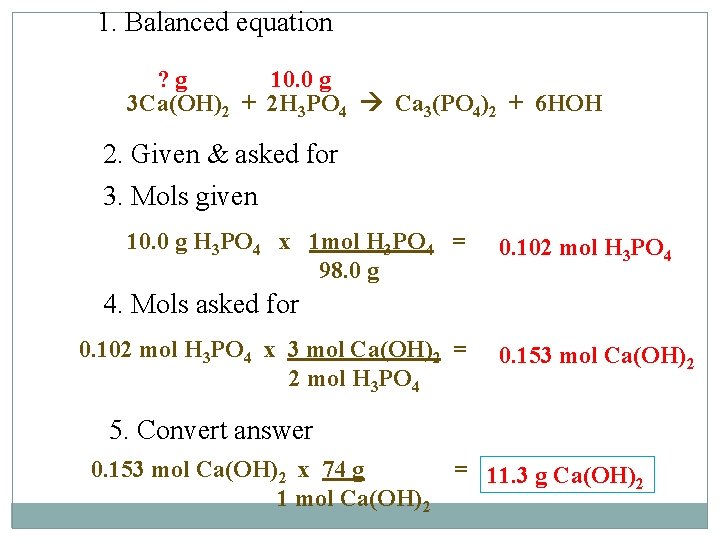
Example #2 How many grams of calcium hydroxide will be needed to react completely with 10. 0 g of phosphoric acid (H 3 PO 4) in a double displacement reaction?

1. Balanced equation ? g 10. 0 g 3 Ca(OH)2 + 2 H 3 PO 4 Ca 3(PO 4)2 + 6 HOH 2. Given & asked for 3. Mols given 10. 0 g H 3 PO 4 x 1 mol H 3 PO 4 = 98. 0 g 0. 102 mol H 3 PO 4 4. Mols asked for 0. 102 mol H 3 PO 4 x 3 mol Ca(OH)2 = 2 mol H 3 PO 4 0. 153 mol Ca(OH)2 5. Convert answer 0. 153 mol Ca(OH)2 x 74 g 1 mol Ca(OH)2 = 11. 3 g Ca(OH)2

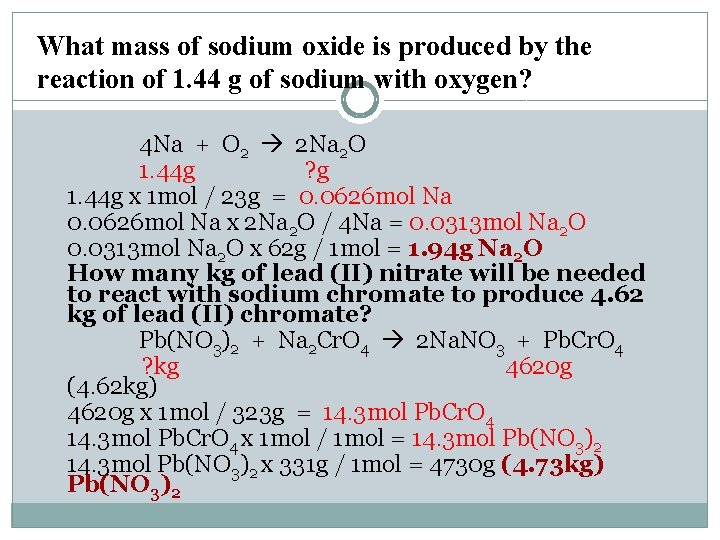
What mass of sodium oxide is produced by the reaction of 1. 44 g of sodium with oxygen? 4 Na + O 2 2 Na 2 O 1. 44 g ? g 1. 44 g x 1 mol / 23 g = 0. 0626 mol Na x 2 Na 2 O / 4 Na = 0. 0313 mol Na 2 O x 62 g / 1 mol = 1. 94 g Na 2 O How many kg of lead (II) nitrate will be needed to react with sodium chromate to produce 4. 62 kg of lead (II) chromate? Pb(NO 3)2 + Na 2 Cr. O 4 2 Na. NO 3 + Pb. Cr. O 4 ? kg 4620 g (4. 62 kg) 4620 g x 1 mol / 323 g = 14. 3 mol Pb. Cr. O 4 x 1 mol / 1 mol = 14. 3 mol Pb(NO 3)2 x 331 g / 1 mol = 4730 g (4. 73 kg) Pb(NO 3)2

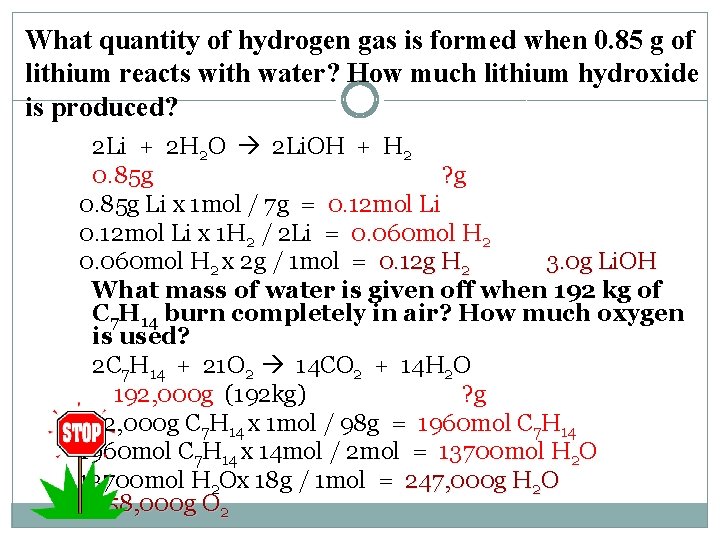
What quantity of hydrogen gas is formed when 0. 85 g of lithium reacts with water? How much lithium hydroxide is produced? 2 Li + 2 H 2 O 2 Li. OH + H 2 0. 85 g ? g 0. 85 g Li x 1 mol / 7 g = 0. 12 mol Li x 1 H 2 / 2 Li = 0. 060 mol H 2 x 2 g / 1 mol = 0. 12 g H 2 3. 0 g Li. OH What mass of water is given off when 192 kg of C 7 H 14 burn completely in air? How much oxygen is used? 2 C 7 H 14 + 21 O 2 14 CO 2 + 14 H 2 O 192, 000 g (192 kg) ? g 192, 000 g C 7 H 14 x 1 mol / 98 g = 1960 mol C 7 H 14 x 14 mol / 2 mol = 13700 mol H 2 Ox 18 g / 1 mol = 247, 000 g H 2 O 658, 000 g O 2

Classwork P. 307, #1 -4 Fe 2 O 3 + 2 Al 2 Fe + Al 2 O 3 P. 309, #1 -4

Homework P. 311, #1 -7