Starter S53 Mole Day Starter S55 What is
















- Slides: 29

Starter S-53 Mole Day!

Starter S-55 What is the value of a mole? What is that number used for?

Chapter 7 Ionic and Metallic Bonding

Chapter 7 7. 1 Ions

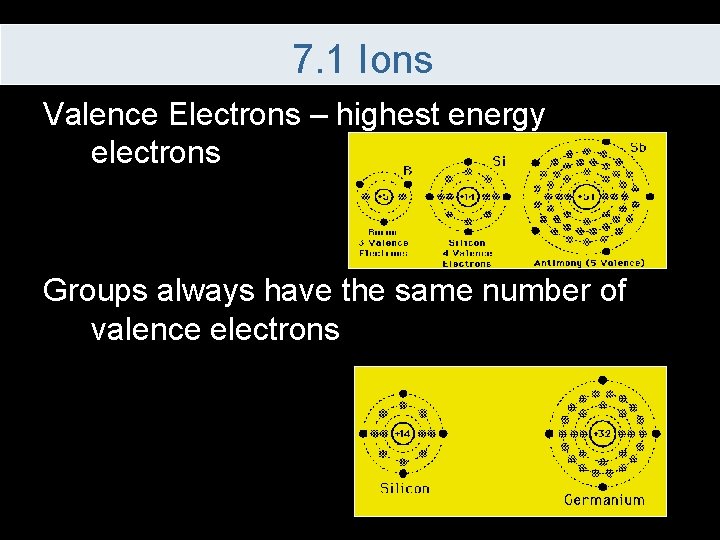
7. 1 Ions Valence Electrons – highest energy electrons Groups always have the same number of valence electrons

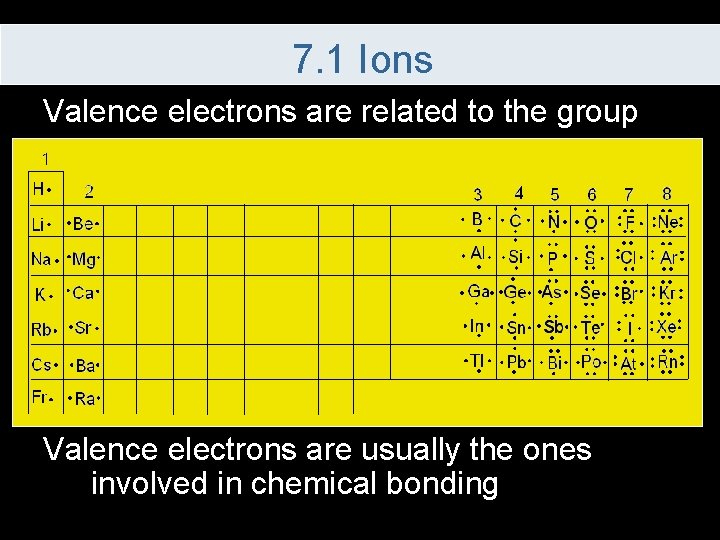
7. 1 Ions Valence electrons are related to the group Valence electrons are usually the ones involved in chemical bonding

7. 1 Ions Octet Rule Metals tend to lose their valence electrons leaving a complete octet in the next lowest energy level Nonmetals tend to gain or share electrons to complete their octet Ionic Bonding

7. 1 Ions Ionic Bonding involves the formation of a Cation – lose electrons Anions – gain electrons Formation of Ions

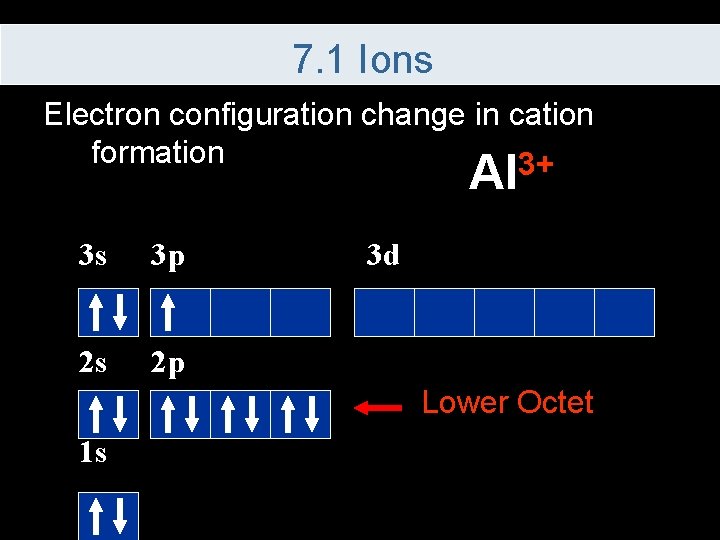
7. 1 Ions Electron configuration change in cation formation 3+ Al 3 s 3 p 2 s 2 p 3 d Lower Octet 1 s

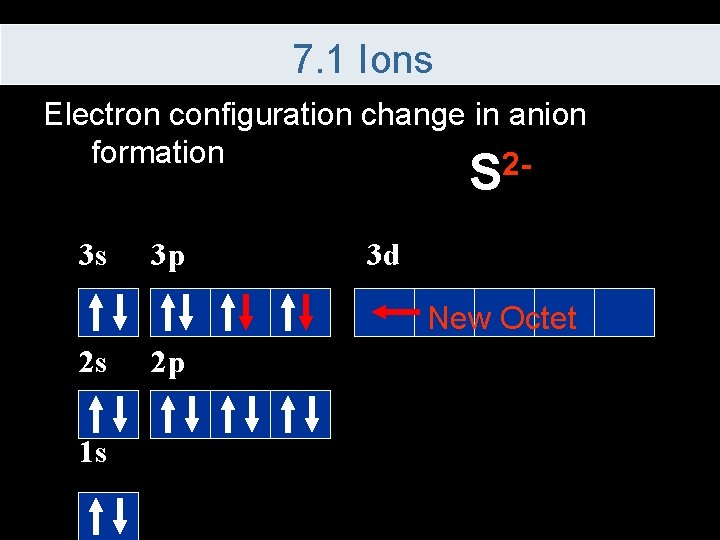
7. 1 Ions Electron configuration change in anion formation 2 - S 3 s 3 p 3 d New Octet 2 s 1 s 2 p

7. 1 Ions Oxidation Number – apparent charge in a compound

Starter S-57 What is an ion? How many electrons does Oxygen need in its outer energy level to be stable? Why does Helium only need two electrons to be stable?

Chapter 7 7. 2 Ionic Bonds and Ionic Compounds

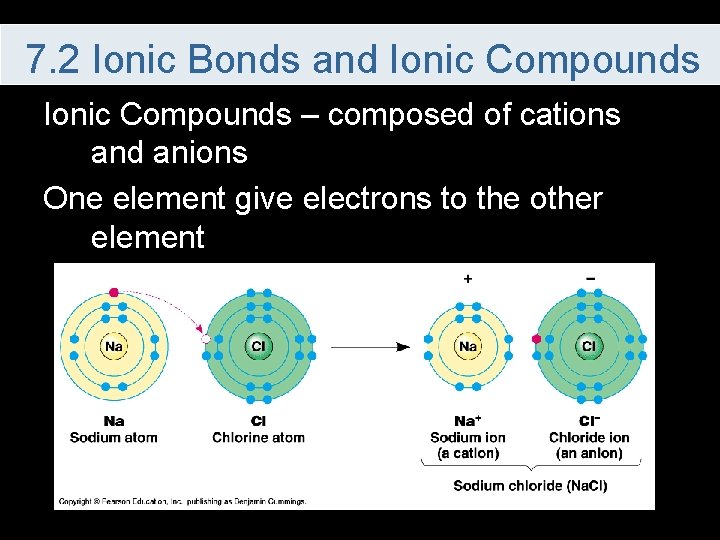
7. 2 Ionic Bonds and Ionic Compounds – composed of cations and anions One element give electrons to the other element

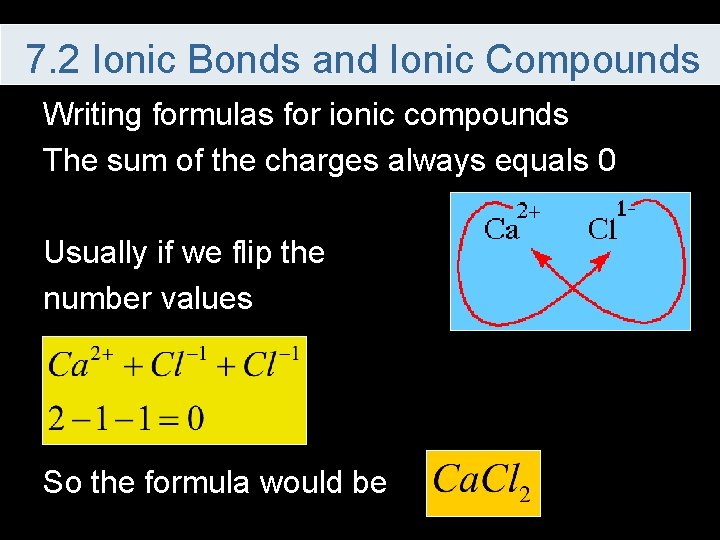
7. 2 Ionic Bonds and Ionic Compounds Writing formulas for ionic compounds The sum of the charges always equals 0 Usually if we flip the number values So the formula would be

7. 2 Ionic Bonds and Ionic Compounds Positive ion is always written first Numbers are written after the element and as a subscript The number 1 is never written

7. 2 Ionic Bonds and Ionic Compounds Try Aluminum and Oxygen Calculations Formula

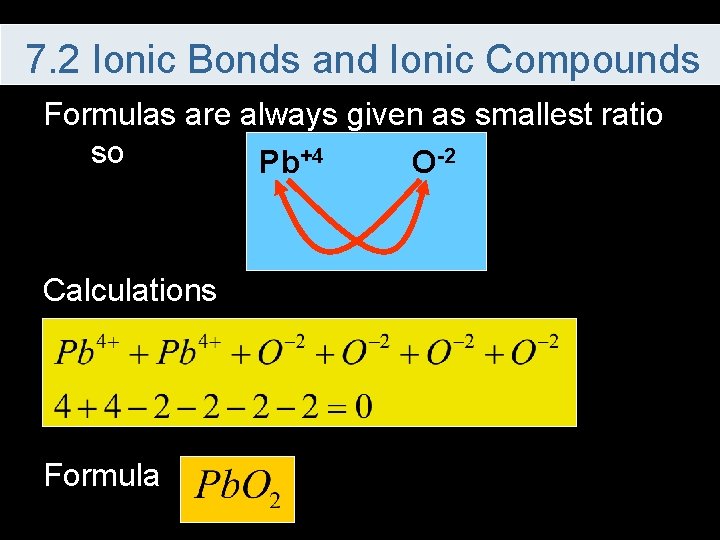
7. 2 Ionic Bonds and Ionic Compounds Formulas are always given as smallest ratio so Pb+4 O-2 Calculations Formula

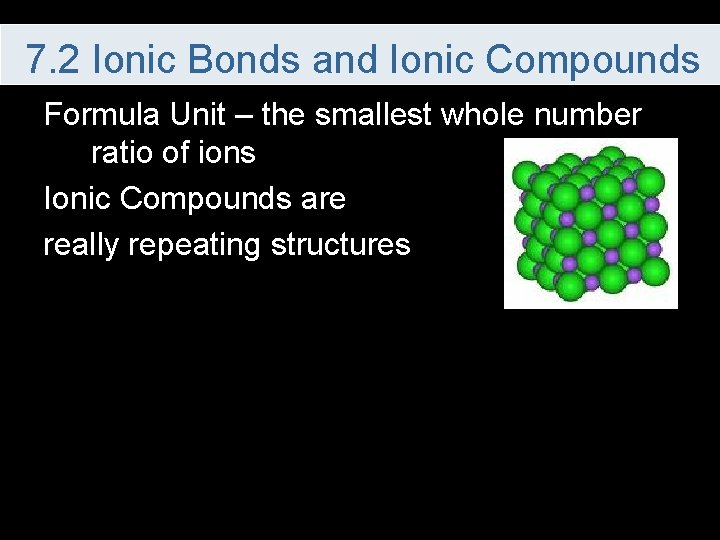
7. 2 Ionic Bonds and Ionic Compounds Formula Unit – the smallest whole number ratio of ions Ionic Compounds are really repeating structures

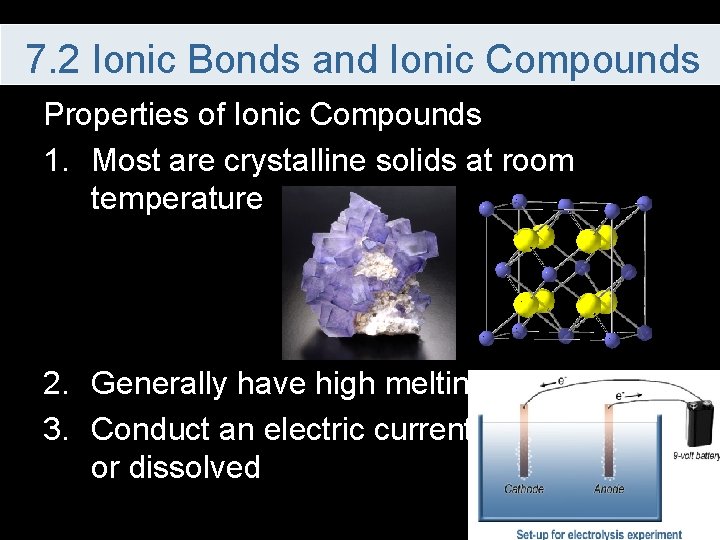
7. 2 Ionic Bonds and Ionic Compounds Properties of Ionic Compounds 1. Most are crystalline solids at room temperature 2. Generally have high melting points 3. Conduct an electric current when melted or dissolved

Starter S-59 What is the formula for 1. Vanadium (VI) and Oxygen 2. Vanadium (V) and Oxygen 3. Lead (IV) and Sulfur 4. Lead (II) and Sulfur

Chapter 7 7. 3 Bonding in Metals

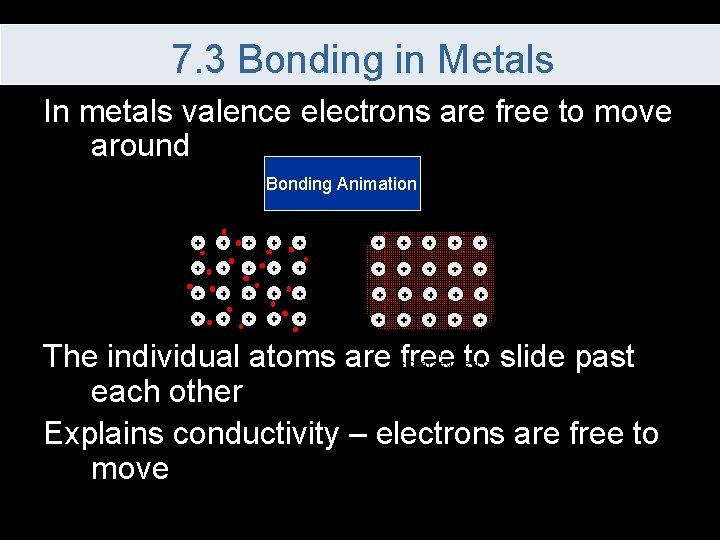
7. 3 Bonding in Metals In metals valence electrons are free to move around Bonding Animation The individual atoms are free to slide past each other Explains conductivity – electrons are free to move

7. 3 Bonding in Metals Ductility and Malleability – metals free to slide past each other Metals are arranged in compact and orderly crystal patterns Crystal Structures Body Centered Cubic-every atom has eight neighbors Na, K, Fe, Cr, W

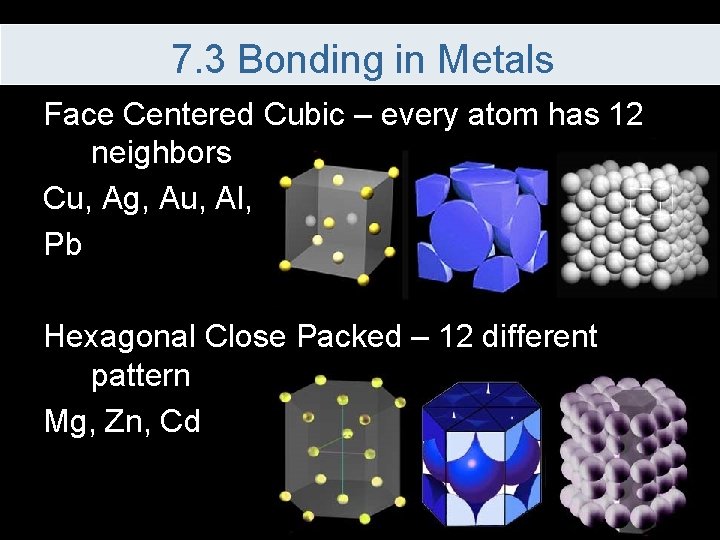
7. 3 Bonding in Metals Face Centered Cubic – every atom has 12 neighbors Cu, Ag, Au, Al, Pb Hexagonal Close Packed – 12 different pattern Mg, Zn, Cd

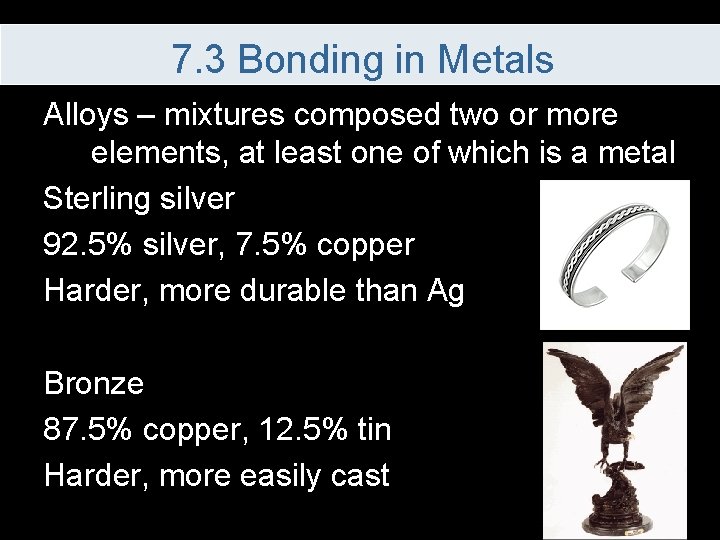
7. 3 Bonding in Metals Alloys – mixtures composed two or more elements, at least one of which is a metal Sterling silver 92. 5% silver, 7. 5% copper Harder, more durable than Ag Bronze 87. 5% copper, 12. 5% tin Harder, more easily cast

7. 3 Bonding in Metals Steel Stainless Steel (80. 6% Fe, 18. 0% Cr, 0. 4% C, 1. 0 % Ni) Spring Steel (98. 6% Fe, 1. 0% Cr, 0. 4% C)

7. 3 Bonding in Metals Surgical Steel (67% Fe, 18% Cr, 12% Ni, 3% Mo)

Starter S-60 What is the chemical formula of A. Iron (III) and Chlorine B. Calcium and Fluorine C. Sodium and Oxygen