STARTER Are all types of radiation bad Are
STARTER Are all types of radiation bad? Are some good? What examples of radiation can you give and explain how they are good or bad? Remember radiation just means an emission of energy, what different types of radiation can you remember? Too much UV light can be dangerous to us as it can damage skin cells, but we can also use UV light to sterilise surgical equipment. Too much exposure to Xrays can cause cells to mutate but we can also use x-rays for medical imaging to help diagnosis and treatment.

Learning objectives 1. Review the properties of the 3 types of nuclear radiation. (D) 2. Identify that nuclear radiation can be dangerous and useful. (C) 3. Explain in detail one or two applications of nuclear radiation. (B) 4. Apply your knowledge of nuclear radiation to exam questions. (A) NOTICES: ……………. Keywords: - Alpha - Beta - Gamma - Half-life - Ionisation - Isotope - Activity
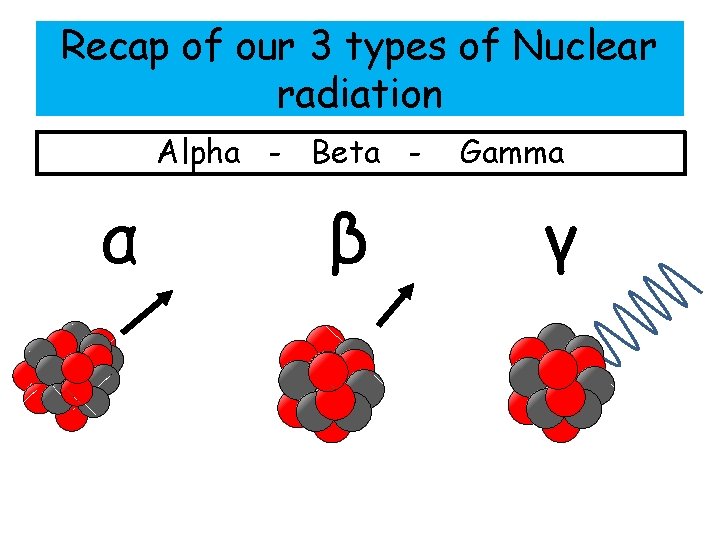
Recap of our 3 types of Nuclear radiation Alpha - Beta - α β Gamma γ
Properties of Nuclear Radiation Complete the table below Type of Radiation Symbol Range in Air Stopped by Ionising ability Alpha Beta Gamma Some help: 5 cm α Concrete Thin paper weak Aluminium 1 m Lead strong unlimited β γ moderate
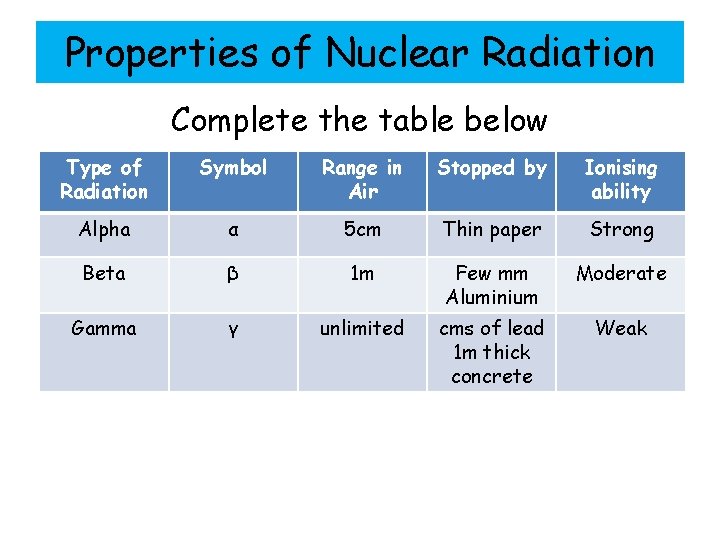
Properties of Nuclear Radiation Complete the table below Type of Radiation Symbol Range in Air Stopped by Ionising ability Alpha α 5 cm Thin paper Strong Beta β 1 m Few mm Aluminium Moderate Gamma γ unlimited cms of lead 1 m thick concrete Weak

Dangers of Radioactivity Nuclear radiation is dangerous due to ionisation (knocking electrons out of atoms, making them charged). Why is this dangerous to living cells? When atoms in living cells become ionized one of three things usually happen – the cell dies, the cell repairs itself, or the cell mutates incorrectly and can become cancerous. If nuclear radiation is dangerous, how comes we use it to treat some cancers?
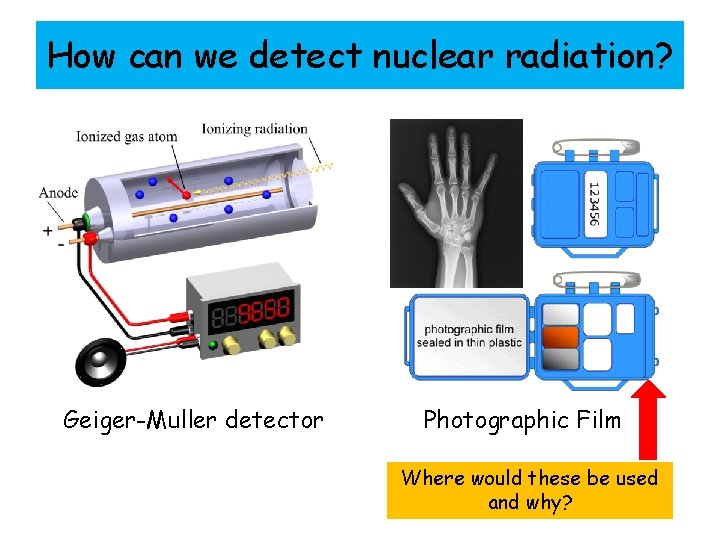
How can we detect nuclear radiation? Geiger-Muller detector Photographic Film Where would these be used and why?

How can we detect nuclear radiation? Photographic film goes darker when it absorbs radiation. Therefore the more radiation the film is exposed to the darker it will appear once developed. Why can you clearly see the bones of the hand in the x-ray and faintly see the flesh around the bones? Photographic Film
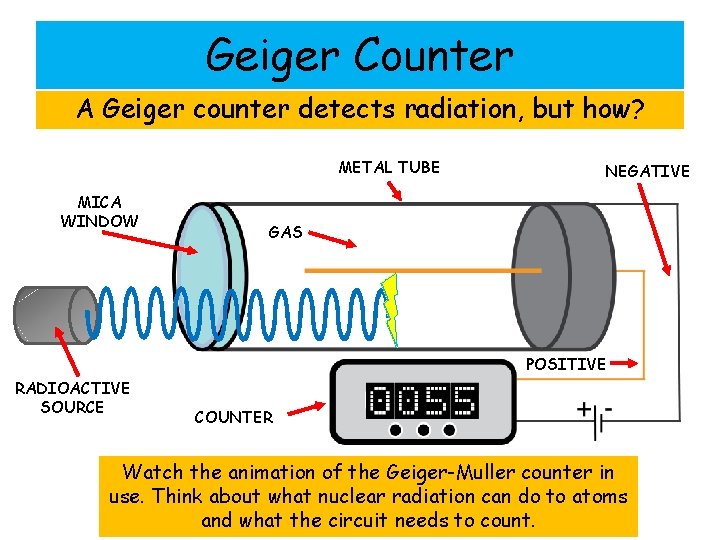
Geiger Counter A Geiger counter detects radiation, but how? METAL TUBE MICA WINDOW NEGATIVE GAS POSITIVE RADIOACTIVE SOURCE COUNTER Watch the animation of the Geiger-Muller counter in use. Think about what nuclear radiation can do to atoms and what the circuit needs to count.
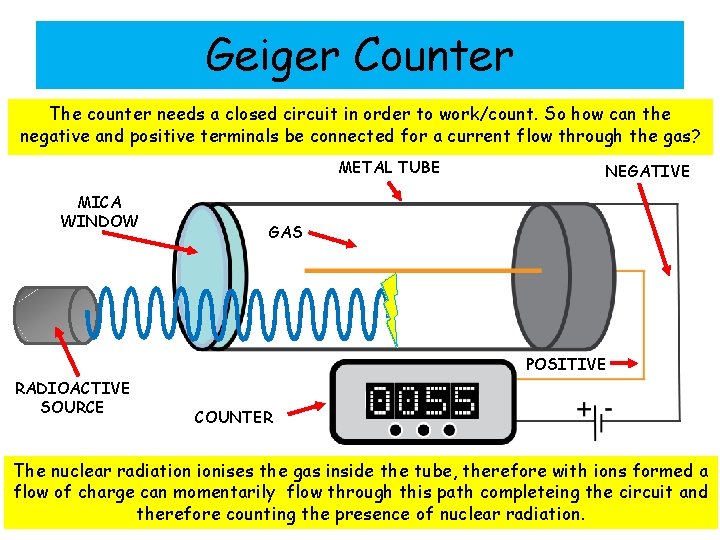
Geiger Counter The counter needs a closed circuit in order to work/count. So how can the negative and positive terminals be connected for a current flow through the gas? METAL TUBE MICA WINDOW NEGATIVE GAS POSITIVE RADIOACTIVE SOURCE COUNTER The nuclear radiation ionises the gas inside the tube, therefore with ions formed a flow of charge can momentarily flow through this path completeing the circuit and therefore counting the presence of nuclear radiation.
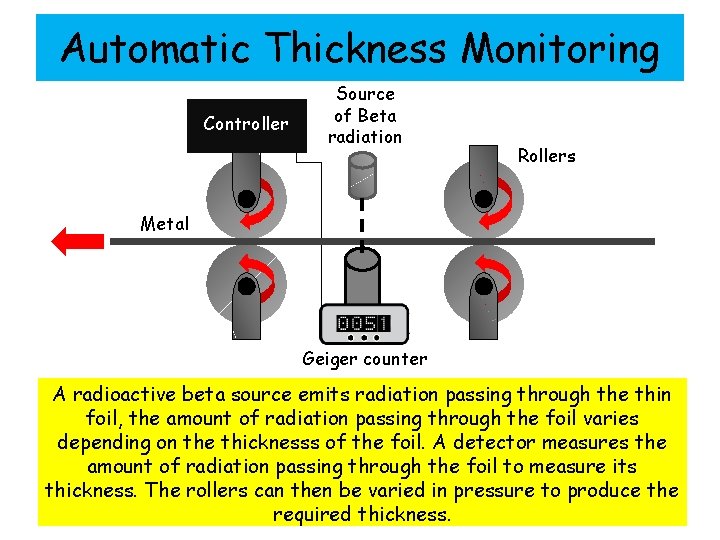
Automatic Thickness Monitoring Controller Source of Beta radiation Rollers Metal Geiger counter A radioactive beta source emits radiation passing through the thin foil, the amount of radiation passing through the foil varies depending on the thicknesss of the foil. A detector measures the amount of radiation passing through the foil to measure its thickness. The rollers can then be varied in pressure to produce the required thickness.
Radioactive Tracers Substances that emit gamma radiation are often used as tracers because the radiation easily passes through many substances. The trace can be consumed into the body and be tracked by a detector as it moves through the body. EXAMPLE: Radioactive iodine can be used to identify a blocked kidney. Once consumed the kidney can be monitored for radioactivity. If detector reads an up and down there is no blockage. If the dectector goes up an stay up a blockage is present. It is important a radioactive substance with a small half-life (a few hours) is used as a trace so it can be tracked through the body and is no longer activity in the body once it is used. Tracers can also be used in industry to: • Find leaks or blockages in underground pipes • Find the route of underground pipes • Track the dispersal of waste
Smoke Alarms Looking at the two diagrams can you explain how a HOW DO SMOKE ALARMS WORK? smoke detector works? Alpha Source + - Current flows due to ionisation of gas Alpha Source + - Current stopped by the smoke Smoke alarms have a radioactive alpha source inside them which is placed between a gap in its circuit. The alpha particles ionise the air allowing the current to flow across the gap. Smoke absorbes the ions needed to allow the current to flow. In the presence of smoke the current inside the smoke alarm drops which triggers the alarm.
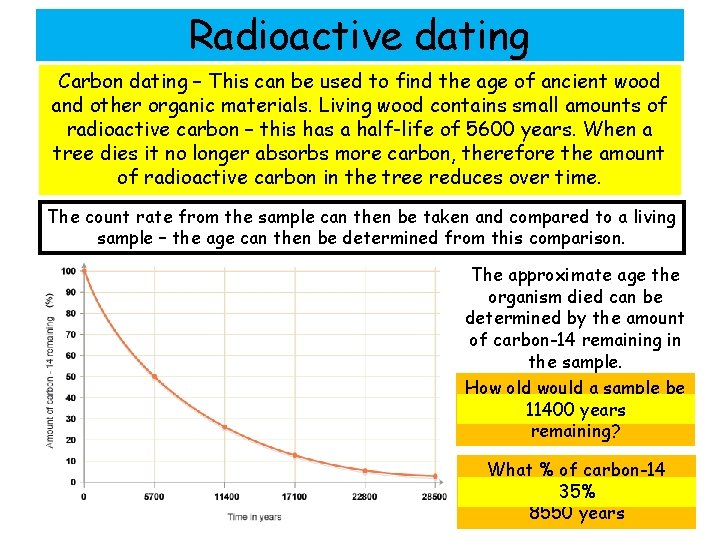
Radioactive dating Carbon dating – This can be used to find the age of ancient wood and other organic materials. Living wood contains small amounts of radioactive carbon – this has a half-life of 5600 years. When a tree dies it no longer absorbs more carbon, therefore the amount of radioactive carbon in the tree reduces over time. The count rate from the sample can then be taken and compared to a living sample – the age can then be determined from this comparison. The approximate age the organism died can be determined by the amount of carbon-14 remaining in the sample. How old would a sample be if it had 25%years Carbon-14 11400 remaining? What % of carbon-14 would be remaining after 35% 8550 years
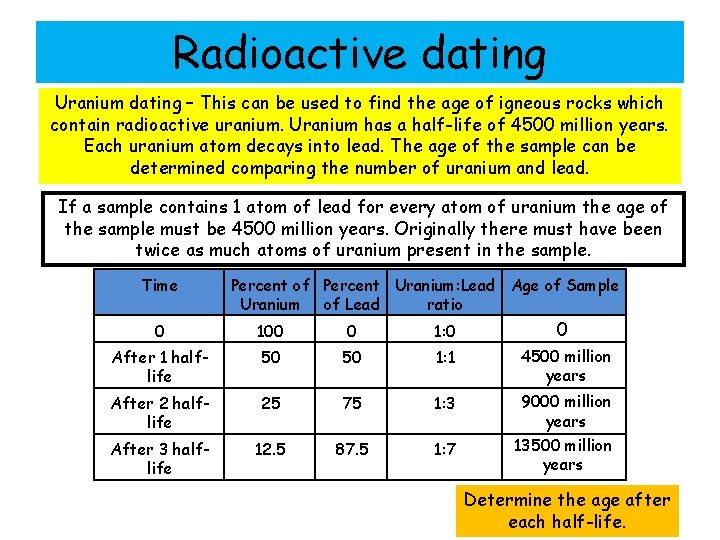
Radioactive dating Uranium dating – This can be used to find the age of igneous rocks which contain radioactive uranium. Uranium has a half-life of 4500 million years. Each uranium atom decays into lead. The age of the sample can be determined comparing the number of uranium and lead. If a sample contains 1 atom of lead for every atom of uranium the age of the sample must be 4500 million years. Originally there must have been twice as much atoms of uranium present in the sample. Time Percent of Percent Uranium: Lead Age of Sample Uranium of Lead ratio 0 100 0 1: 0 0 After 1 halflife 50 50 1: 1 4500 million years After 2 halflife 25 75 1: 3 9000 million years After 3 halflife 12. 5 87. 5 1: 7 13500 million years Determine the age after each half-life.

Review of Nuclear Radiation Properties 1) A 1. Geiger-muller requires gas tocounter ionise allowing the circuit be closed andno therefore Would counter a geiger-muller work if thetotube had gas incount it radioactivity. (1) (a vacuum)? (3) 2) Gamma radiation easily passes through thin Aluminium so would not vary in detection (1) 2. Would a gamma source be suitable for automatic thickness of aluminium foil? Explain yourradiation answer. 3)monitoring A thickness of 5 mm+ is required to completely stop Beta (1) Variations in thickness of Aluminium would vary the amount of beta radiation passing through the foil 3. Beta radiation can bewhich stopped by Aluminium, how comes it can is detectable (1) be used for monitoring thickness of aluminium foil? (2) 4) Alpha radiation is highly ionising (1) 4. Why is an alpha source most appropriate for use in a smoke Alpha radiation has a short range in air so poses not health risk as it is contained in the detector (1) detector? (2) 5) Uranium decays to lead (1) 5. Explain how the. The age of anofigneous can be determined by half-life uranium is rock known (1) The ratio of uranium to lead atoms present can be determined in the sample (1) Uranium dating? (4) The age can be determined by comparing the uranium/lead ratio to the uraniums half-life (1) 6. Why would the age of rock not be possible to determine using a 6) The half-life of the source is too short (1) radioactive isotope with a half-life of 15 hours? (2) The isotope would have decayed long ago and be hard to read against background radiation (1) 7) Nuclear radiation is ionising (1) 7. damage Explain why nuclear radiation can be dangerous? Can cells/mutate cells/cause cancer or tumours/cannot be seen (1) 8. Explain whycan a be radioactive gamma sourcethrough is with half 8) Gamma detected outside the body/pass the a body (1) life of 6 Half-life is long enough to be detectable as it moves through body (1)(3) hours is suitable for medical imaging as athe tracer. Half-life short enough to fall to low levels of activity soon after use (1)
- Slides: 16