Stanton Rowe CEO NXT Biomedical The Feasibility Cunundrum

Stanton Rowe CEO NXT Biomedical The Feasibility Cunundrum

DISCLOSURE STATEMENT OF FINANCIAL INTEREST Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below AFFILIATION/FINANCIAL RELATIONSHIP COMPANY • Consulting Fees • Shareholder/Equity • Edwards Lifesciences

What drives us? • We are want to develop products that are impactful and improve the lives of patients worldwide.


Your idea… • How do you know that the device/therapy you propose is worth the effort? • How do you objectively and quantitatively assess the market opportunity? • How much improvement in outcomes/performance justifies the risk/cost/time of development? • Is iterative development (outside of big med device) worth it?
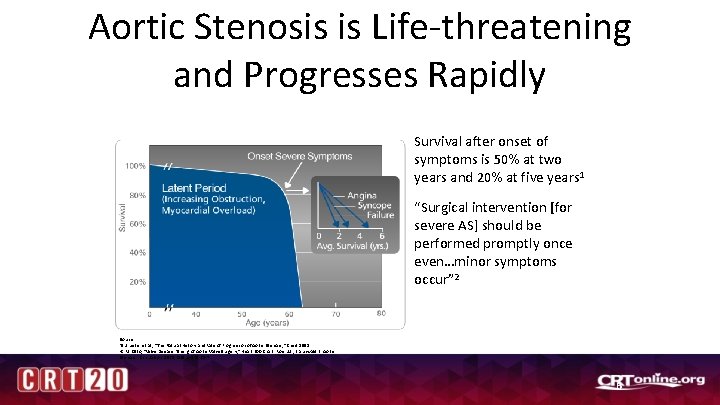
Aortic Stenosis is Life-threatening and Progresses Rapidly Survival after onset of symptoms is 50% at two years and 20% at five years 1 “Surgical intervention [for severe AS] should be performed promptly once even…minor symptoms occur” 2 Source: 1 S. J. Lester et al. , “The Natural History and Rate of Progression of Aortic Stenosis, ” Chest 1998. 2 C. M. Otto, “Valve Disease: Timing of Aortic Valve Surgery, ” Heart 200 Chart: Ross J Jr, Braunwald E. Aortic Stenosis. Circulation. 1968; 38(Suppl 1): 61 -7. 6
Current Therapy- Open Heart Surgery
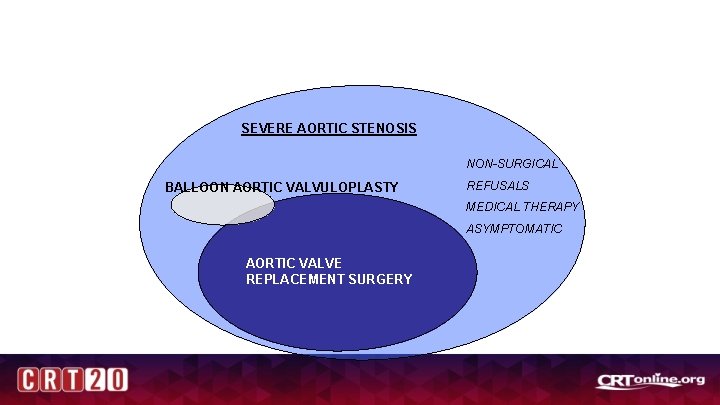
SEVERE AORTIC STENOSIS NON-SURGICAL BALLOON AORTIC VALVULOPLASTY REFUSALS MEDICAL THERAPY ASYMPTOMATIC AORTIC VALVE REPLACEMENT SURGERY
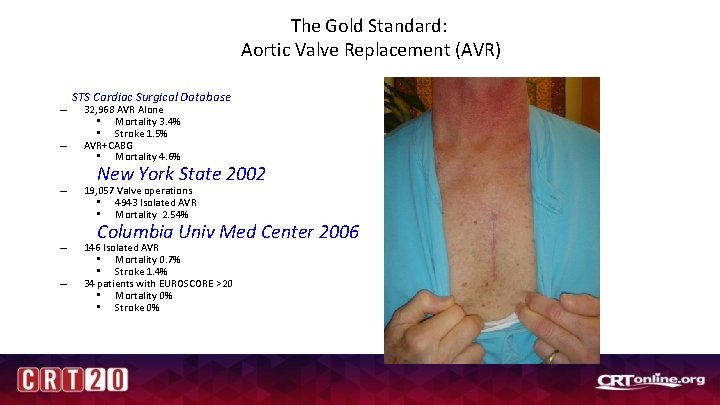
The Gold Standard: Aortic Valve Replacement (AVR) – – STS Cardiac Surgical Database 32, 968 AVR Alone • Mortality 3. 4% • Stroke 1. 5% AVR+CABG • Mortality 4. 6% New York State 2002 – 19, 057 Valve operations • 4943 Isolated AVR • Mortality 2. 54% – 146 Isolated AVR • Mortality 0. 7% • Stroke 1. 4% 34 patients with EUROSCORE >20 • Mortality 0% • Stroke 0% – Columbia Univ Med Center 2006
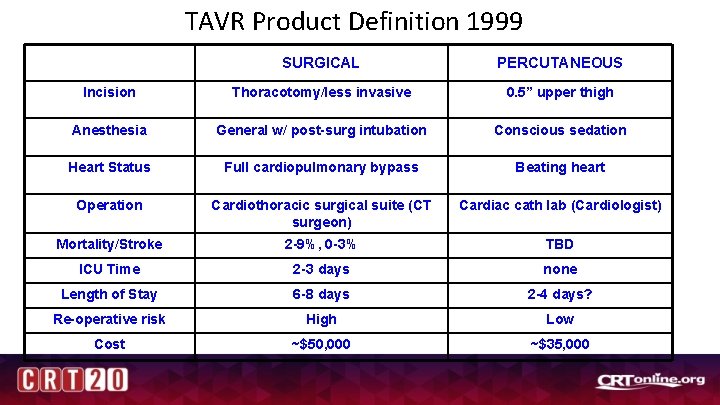
TAVR Product Definition 1999 SURGICAL PERCUTANEOUS Incision Thoracotomy/less invasive 0. 5” upper thigh Anesthesia General w/ post-surg intubation Conscious sedation Heart Status Full cardiopulmonary bypass Beating heart Operation Cardiothoracic surgical suite (CT surgeon) Cardiac cath lab (Cardiologist) Mortality/Stroke 2 -9%, 0 -3% TBD ICU Time 2 -3 days none Length of Stay 6 -8 days 2 -4 days? Re-operative risk High Low Cost ~$50, 000 ~$35, 000
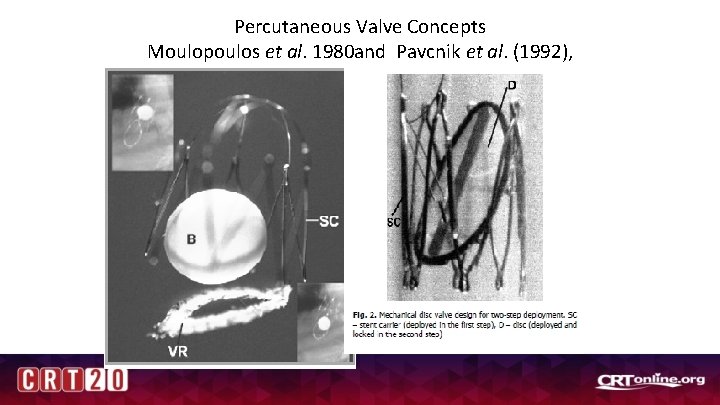
Percutaneous Valve Concepts Moulopoulos et al. 1980 and Pavcnik et al. (1992),

Percutaneous Valve Technologies, Inc. (PVT) ØIncorporated 1999 Founding Partners Dr. Alain Cribier Dr. Martin Leon Stanley Rabinovich Stanton Rowe ØMay-2000 – Angels, founders and Aran funded 9 months

• You need great partners and early funding from Angels • Define feasibility; different for each product • For TAVR, it was: – Benchtop durability and hemodynamics – Chronic animal studies success – FIH n=3
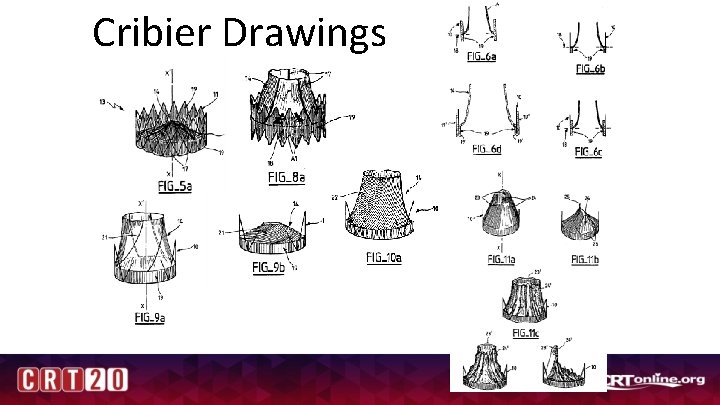
Cribier Drawings

Engineering questions • • • What compressive forces must the frame (stent) resist? How strong must we make the frame to form a circular valve? How can new manufacture a frame that large; no tubing that large? What material is preferred for the frame? How do we attach a fixed diameter valve to an expandable and collapsible frame? How do you make the attachment durable? How can we seal around the valve and prevent PVL? Without increasing profile? What is the optimal valve design for hemodynamics/profile/tissue damage? Unicuspid, bicuspid, tricuspid or quadracuspid? What is the optimal valve material? Polymers, co-polymers, tissue?
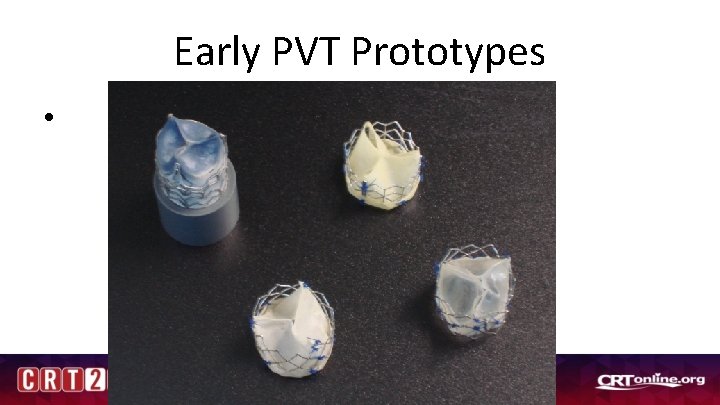
Early PVT Prototypes •
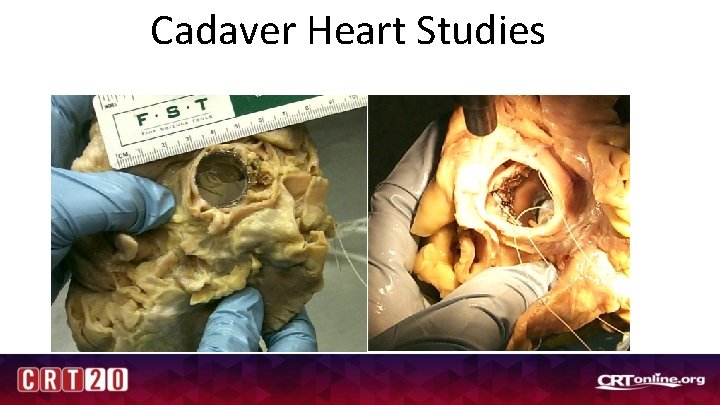
Cadaver Heart Studies

Percutaneous Valve Technologies

• You need amazing, first rate, experienced engineering talent.

Crimped Percutaneous Valve
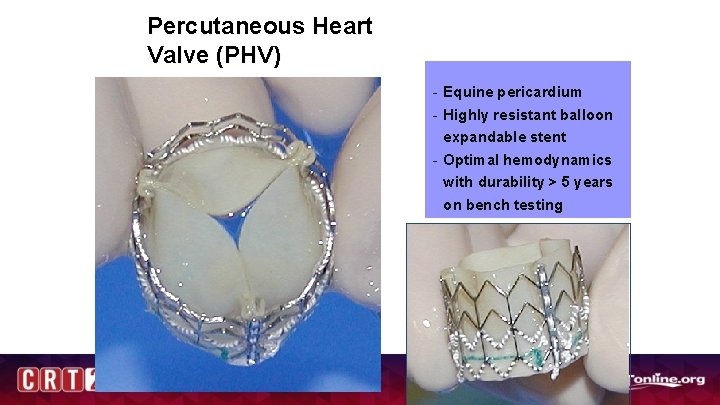
Percutaneous Heart Valve (PHV) - Equine pericardium - Highly resistant balloon expandable stent - Optimal hemodynamics with durability > 5 years on bench testing
Heroic Therapy

Feasibility is the first critical step; what is the conundrum? • Someone must believe in your story to fund early feasibility…BEFORE you have feasibility! Yes, a conundrum. • Your idea needs to address significant unmet needs to justify the risk, cost of capital and years of development. • You must define feasibility carefully for each new product, just like you would define the outcomes you would measure in a clinical trial. • You need a very capable experienced development team with diverse backgrounds. • You will fail and adapt multiple times through feasibility… 23

It’s About Patients!
- Slides: 24