Standards of Measurement PG 7 Units Standards and





- Slides: 11

Standards of Measurement PG. 7

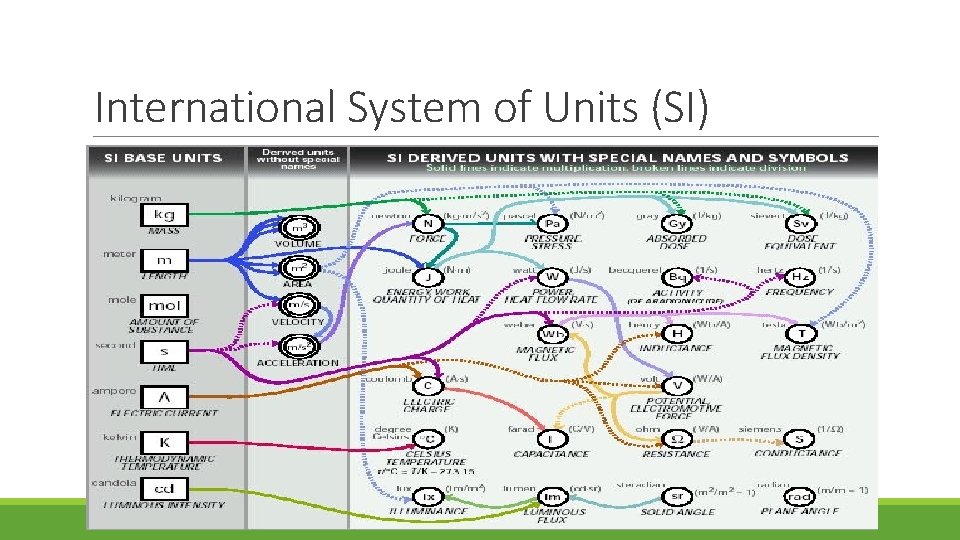
Units, Standards, and Prefixes A standard is an exact quantity that people agree to use to compare measurements. Known as the International System of Units (SI), these standards are universally accepted and understood by scientists throughout the world. Each type of SI measurement has a base unit. The SI system is easy to use because it is based on multiples of ten. Prefixes are used with the names of the units to indicate what multiple of ten should be used with the units.

International System of Units (SI)

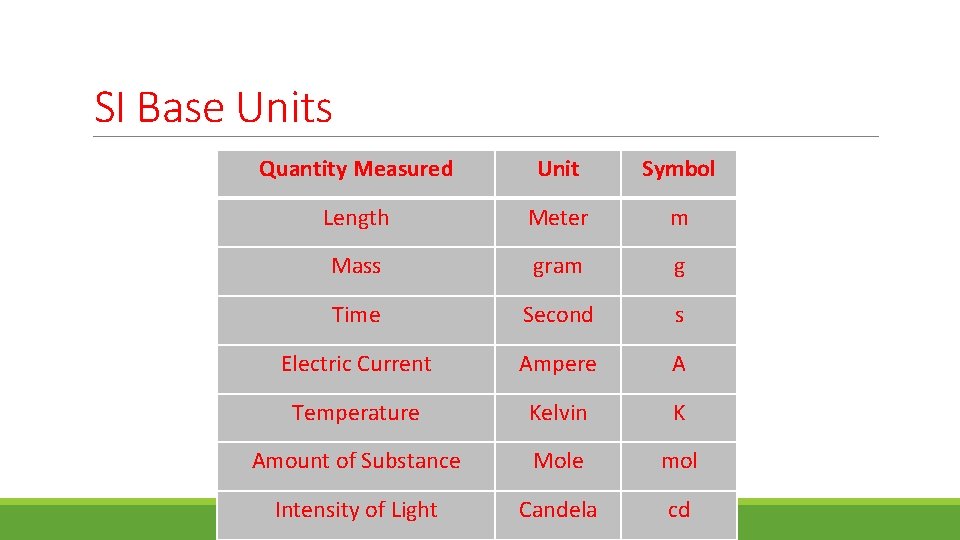
SI Base Units Quantity Measured Unit Symbol Length Meter m Mass gram g Time Second s Electric Current Ampere A Temperature Kelvin K Amount of Substance Mole mol Intensity of Light Candela cd

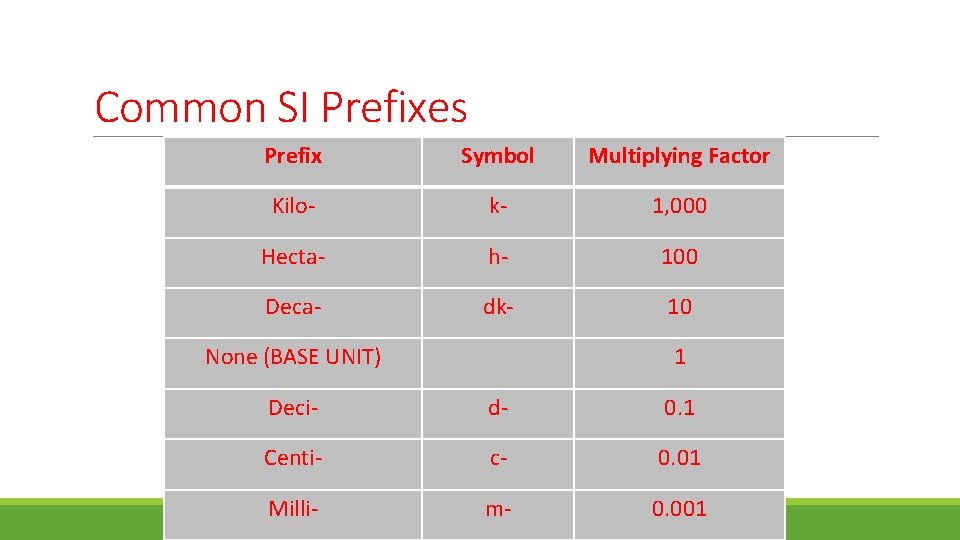
Common SI Prefixes Prefix Symbol Multiplying Factor Kilo- k- 1, 000 Hecta- h- 100 Deca- dk- 10 None (BASE UNIT) 1 Deci- d- 0. 1 Centi- c- 0. 01 Milli- m- 0. 001

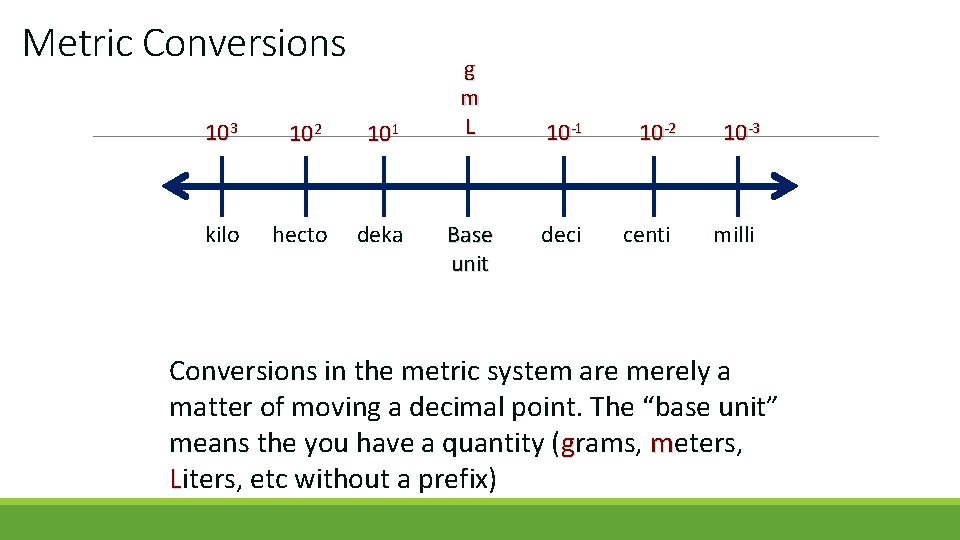
Metric Conversions 103 102 101 kilo hecto deka g m L Base unit 10 -1 10 -2 10 -3 deci centi milli Conversions in the metric system are merely a matter of moving a decimal point. The “base unit” means the you have a quantity (grams, meters, Liters, etc without a prefix)

Converting Between SI Units A conversion factor is a ratio that is equal to one and is used to change one unit to another. 1. 255 L x 1, 000 m. L = 1, 255 m. L 1 L

Measurements Length: distance between two points Volume: the amount of space occupied by an object ◦ ◦ ◦ Cylinder= πr 2 Sphere= 4/3πr 2 Square pyramid=1/3 bh Cone=1/3πr 2 h Solid rectangle V=l x w x h Liquid volume 1 m. L=1 cm 3 Mass: measurement of quantity of matter in an object Density: mass per unit volume of material (g/cm 3) **derived unit—combination of SI units** Time: interval between two events Temperature: measure of how hot or cold something is

Measuring Volume Always read volume from the bottom of the meniscus at eye level. The meniscus is the curved surface of a liquid in a narrow cylindrical container. Read the volume using all certain digits and one uncertain digit. ◦ The certain digits are determined from the calibration marks on the cylinder. ◦ The uncertain digit (the last digit of the reading) is estimated. The volume on the right is 52. 7 m. L

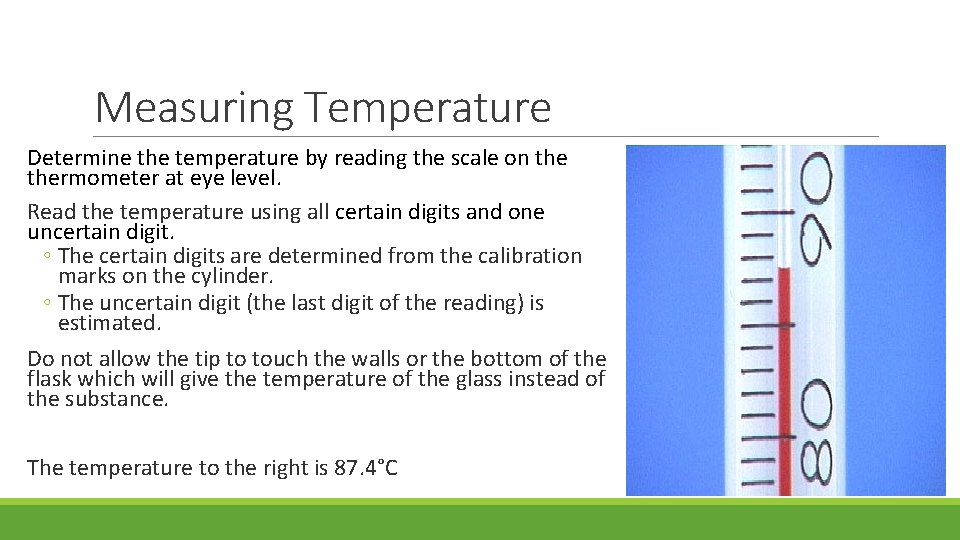
Measuring Temperature Determine the temperature by reading the scale on thermometer at eye level. Read the temperature using all certain digits and one uncertain digit. ◦ The certain digits are determined from the calibration marks on the cylinder. ◦ The uncertain digit (the last digit of the reading) is estimated. Do not allow the tip to touch the walls or the bottom of the flask which will give the temperature of the glass instead of the substance. The temperature to the right is 87. 4°C

Measuring Mass Always check that the balance is level and zeroed before using it. Never weigh directly on the balance pan. Always use a piece of weighing paper to protect it. Do not weigh hot or cold objects. Determine the mass by reading the riders on the beams at eye level. Read the mass by using all certain digits and one uncertain digit. The mass to the right is 114. 49 g