Standardization on Medical Devices Deepak Aggarwal Scientist E










- Slides: 12


Standardization on Medical Devices Deepak Aggarwal Scientist E (Medical Equipment and Hospital Planning Dept. ) Bureau of Indian Standards mhd@bis. gov. in; www. bis. gov. in 14 th December 2018

About BIS Bureau of Indian Standards (BIS), the National Standards Body of India Brief History Set up on 6 January 1947 as Indian Standards Institution for promoting harmonious development of the activities of Ø standardization Ø marking and quality certification of products and services Bureau of Indian standards (BIS) is governed by BIS Act 2016 under Department of Consumer Affairs, Government of India with various objectives including : - Ø Ø providing safe reliable quality goods; minimizing health hazards to consumers; promoting exports and imports substitute; control over proliferation of varieties etc. through standardization, certification and testing. Through this change over, the government envisaged building a climate for quality culture and consciousness and greater participation of consumers in formulation and implementation of national standards.

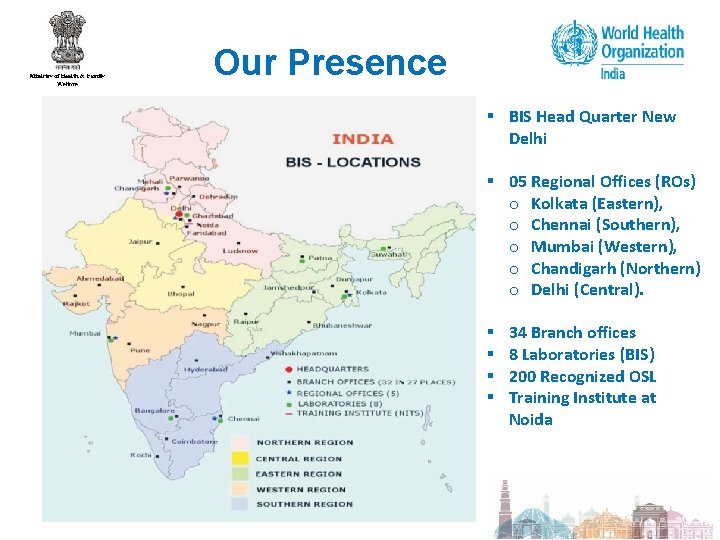
Our Presence § BIS Head Quarter New Delhi § 05 Regional Offices (ROs) o Kolkata (Eastern), o Chennai (Southern), o Mumbai (Western), o Chandigarh (Northern) o Delhi (Central). § § 34 Branch offices 8 Laboratories (BIS) 200 Recognized OSL Training Institute at Noida

BIS Activities In the interest of consumers as well as the industry, BIS is involved in various activities as given below: • • • Standards Formulation Product Certification Scheme Compulsory Registration Scheme Foreign Manufacturers Certification Scheme Hall Marking Scheme Laboratory Services Laboratory Recognition Scheme Sale of Indian Standards Consumer Affairs Activities Promotional Activities Training Services, National & International level

Benefits of Standards in Promoting Quality • • Fitness for Purpose Compatibility Interchangeability Variety Control Safety Protection of the Environment Product Protection (ISO GUIDE 2)

Medical Equipment and Hospital Planning Department (MHD) Setting National Standards on Medical Devices 19 sectional committees, 1200 plus Indian Standards encompass product specifications in various branches of medicine Standardization – A dynamic process Involvement of stakeholders – Manufacturers, clinicians and patients, regulators, health care providers, testing Laboratories - through consensus principle Harmonization with International Standards – ISO/IEC facilitating global trade and thus rendering the new technology accessible and affordable

National Standards on Medical Devices 27 33 MHD 19 Diagnostic Kits MHD 17 Health Informatics 61 MHD 15 Electromedical Eqpt Total Standards published = 1207 Harmonized Standards = 315 23 17 MHD 14 Hospital planning MHD 13 Veterinary 120 MHD 12 Hospital Equipment 54 MHD 11 Anaesthesia 32 MHD 10 laboratory instruments 104 MHD 09 Assistive Products 171 MHD 08 Dentistry 36 45 MHD 07 Neurosurgery MHD 06 cardiovascular 91 MHD 05 Opthalmology 81 MHD 04 ENT 64 MHD 03 gyanecology 132 MHD 02 Orthopedics 112 MHD 01 Surgical instruments 0 50 100 150 200

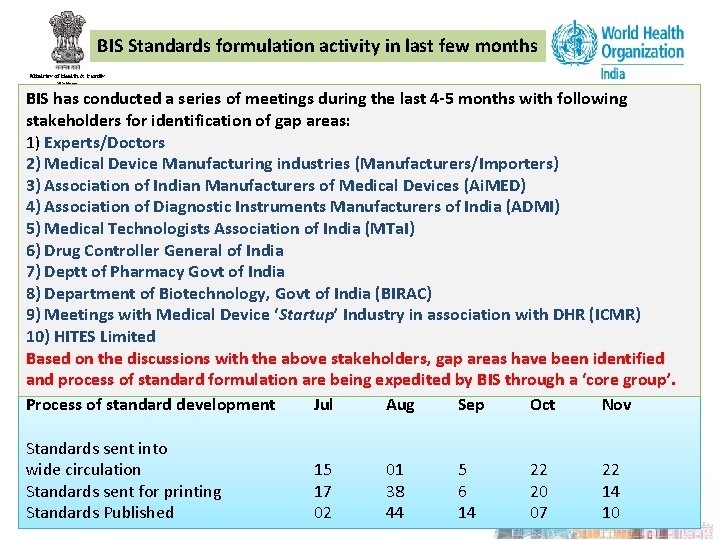
BIS Standards formulation activity in last few months BIS has conducted a series of meetings during the last 4 -5 months with following stakeholders for identification of gap areas: 1) Experts/Doctors 2) Medical Device Manufacturing industries (Manufacturers/Importers) 3) Association of Indian Manufacturers of Medical Devices (Ai. MED) 4) Association of Diagnostic Instruments Manufacturers of India (ADMI) 5) Medical Technologists Association of India (MTa. I) 6) Drug Controller General of India 7) Deptt of Pharmacy Govt of India 8) Department of Biotechnology, Govt of India (BIRAC) 9) Meetings with Medical Device ‘Startup’ Industry in association with DHR (ICMR) 10) HITES Limited Based on the discussions with the above stakeholders, gap areas have been identified and process of standard formulation are being expedited by BIS through a ‘core group’. Process of standard development Jul Aug Sep Oct Nov Standards sent into wide circulation Standards sent for printing Standards Published 15 17 02 01 38 44 5 6 14 22 20 07 22 14 10

Collaborative approach with stakeholders • All stakeholders should implement standards in their dayto-day use for bringing quality in their respective applications. • Active participation of all stakeholders in standard formulation process enables upgradation of standards from time to time. • New items for standardization can be proposed by the Stakeholders on BIS website. • Conformity Assessment schemes of BIS ensure quality products and effective implementation of Standards.

Recommendations for National Governments • Strengthen existing harmonization/alignment process through synchronized efforts of stakeholders including regulatory agencies. • Boost local/regional production of medical devices based on specific national requirements conforming to national standards. • Setting up state of the art laboratories for conformity assessment of medical devices. • Strengthen the use of existing National Standards through regulatory framework and push for their strict compliance in licensing and certification process thus ensuring quality products reaching the customer

Recommendations for WHO/International Organizations • Identification of gap area and a focused approach for standardization requirements in health sector pertaining to developing countries • Standards can be a complementary tool in the efforts of WHO for promoting quality ecosystem of medical devices especially in the developing countries • WHO should continue to provide such platforms for uniformity of implementation of the latest & globally acceptable guidelines on medical devices. • WHO should consider enhancing their promotional activities in developing nations to encourage compliance and reduce the burden of enforcement to curb the menace of substandard medical devices. Thank You