STANDARD MOLAR ENTHALPY OF FORMATION Enthalpy change when


- Slides: 14

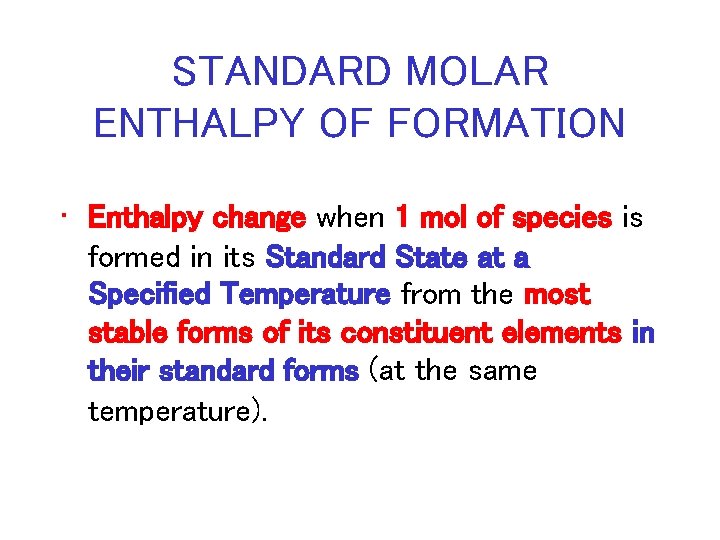
STANDARD MOLAR ENTHALPY OF FORMATION • Enthalpy change when 1 mol of species is formed in its Standard State at a Specified Temperature from the most stable forms of its constituent elements in their standard forms (at the same temperature).

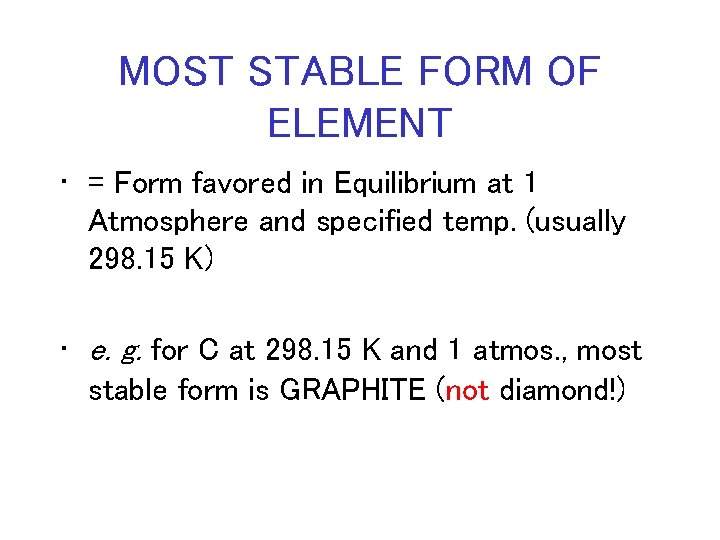
MOST STABLE FORM OF ELEMENT • = Form favored in Equilibrium at 1 Atmosphere and specified temp. (usually 298. 15 K) • e. g. for C at 298. 15 K and 1 atmos. , most stable form is GRAPHITE (not diamond!)

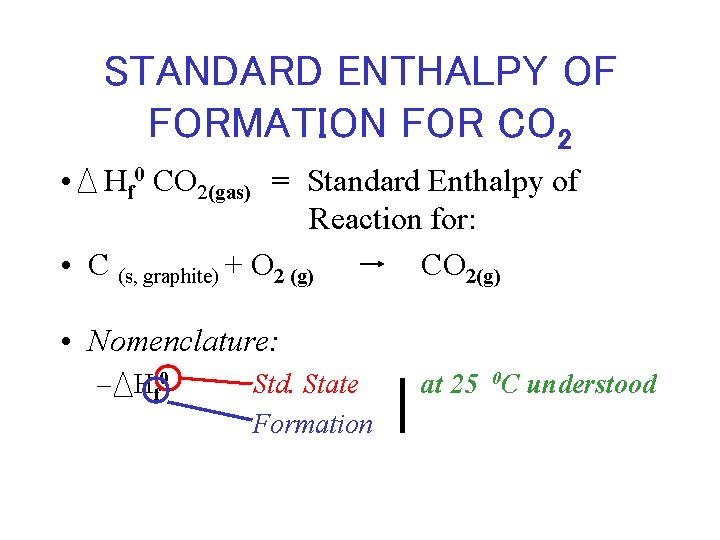
STANDARD ENTHALPY OF FORMATION FOR CO 2 • Hf 0 CO 2(gas) = Standard Enthalpy of Reaction for: • C (s, graphite) + O 2 (g) CO 2(g) • Nomenclature: – H f 0 Std. State Formation at 25 0 C understood

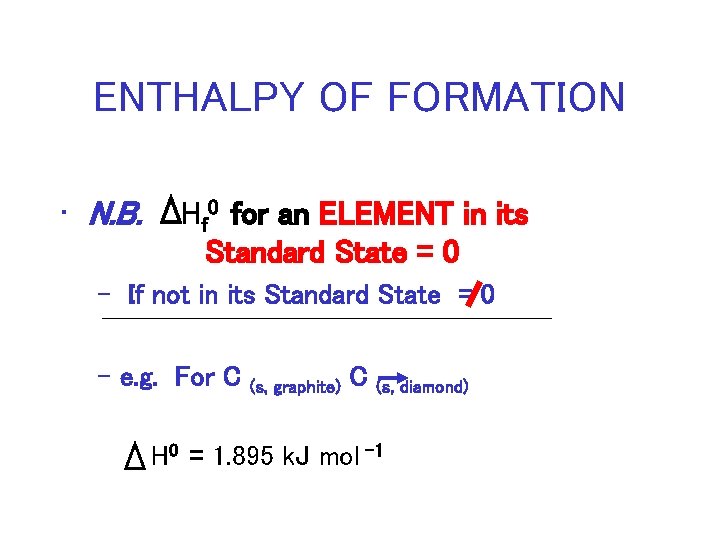
ENTHALPY OF FORMATION • N. B. Hf 0 for an ELEMENT in its Standard State = 0 – If not in its Standard State = 0 – e. g. For C (s, graphite) C (s, diamond) H 0 = 1. 895 k. J mol – 1

CALCULATION OF H FROM TABLE OF H VALUES • 1. Break down steps of reaction into: – (a) Decomposition of Reactants into Elements in Standard Forms – (b) Formation of Products from Elements in Standard States – 2. Apply HESS’S LAW

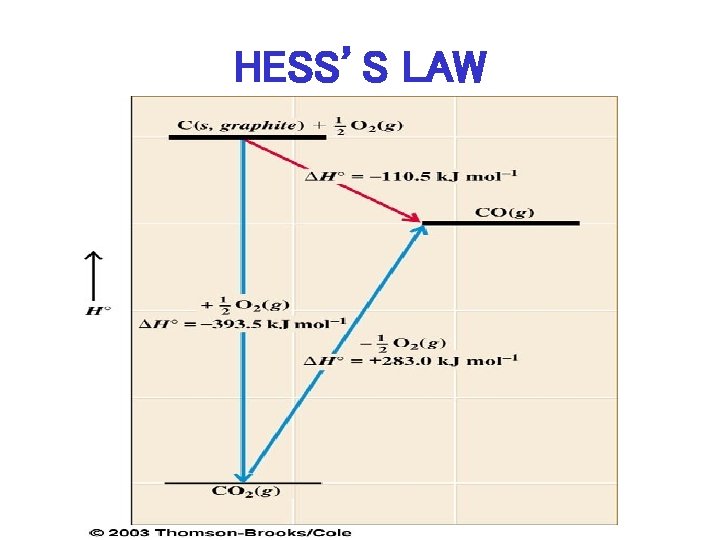
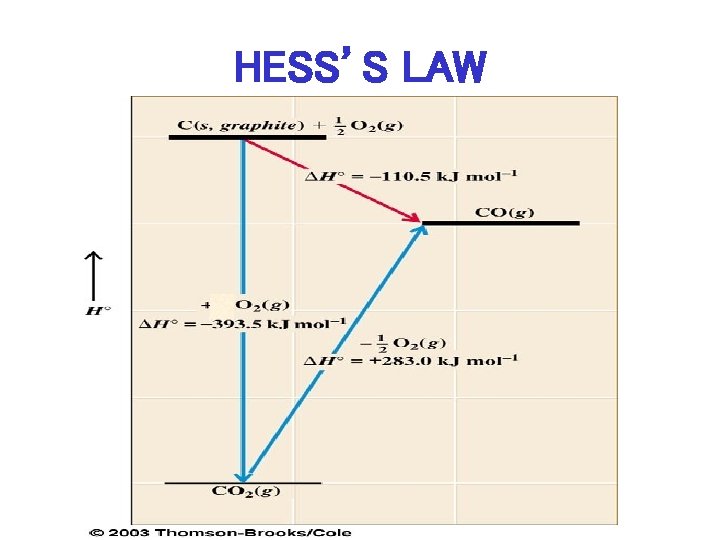
HESS’S LAW Add 2 (or more) Reactions to give New Reaction, then Add Enthalpies in same manner to give Enthalpy of New Reaction

HESS’S LAW

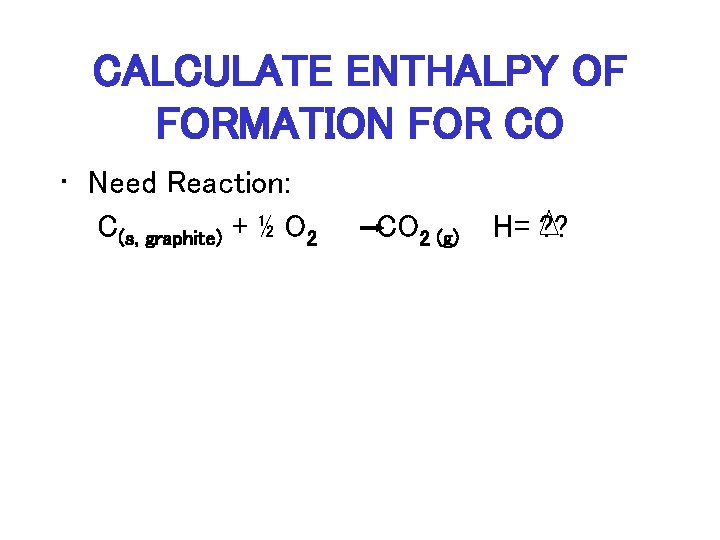
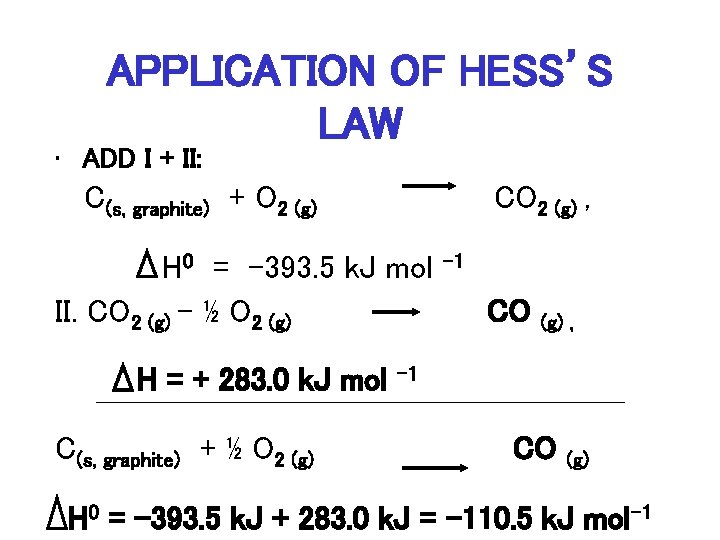
CALCULATE ENTHALPY OF FORMATION FOR CO • Need Reaction: C(s, graphite) + ½ O 2 CO 2 (g) H= ? ?

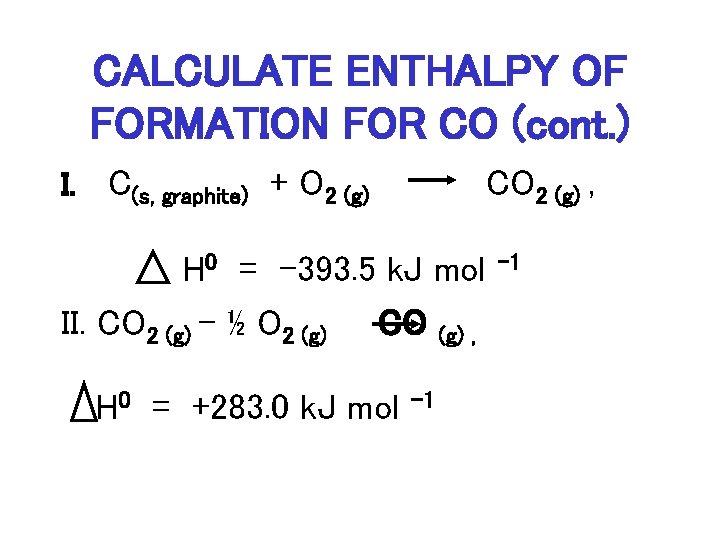
CALCULATE ENTHALPY OF FORMATION FOR CO (cont. ) I. C(s, graphite) + O 2 (g) CO 2 (g) , H 0 = -393. 5 k. J mol II. CO 2 (g) – ½ O 2 (g) CO (g) , H 0 = +283. 0 k. J mol – 1

APPLICATION OF HESS’S LAW • ADD I + II: C(s, graphite) + O 2 (g) CO 2 (g) , H 0 = -393. 5 k. J mol II. CO 2 (g) – ½ O 2 (g) H = + 283. 0 k. J mol C(s, graphite) + ½ O 2 (g) – 1 CO (g) , – 1 CO (g) H 0 = -393. 5 k. J + 283. 0 k. J = -110. 5 k. J mol-1

HESS’S LAW

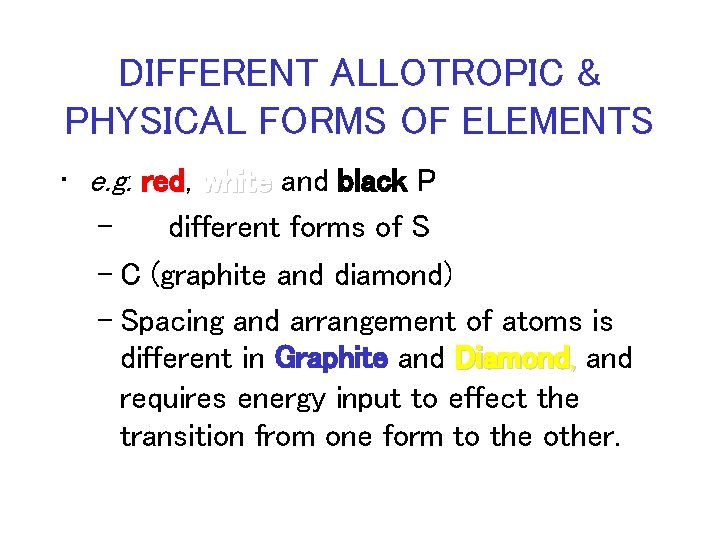
DIFFERENT ALLOTROPIC & PHYSICAL FORMS OF ELEMENTS • e. g. red, white and black P – different forms of S – C (graphite and diamond) – Spacing and arrangement of atoms is different in Graphite and Diamond, and requires energy input to effect the transition from one form to the other.

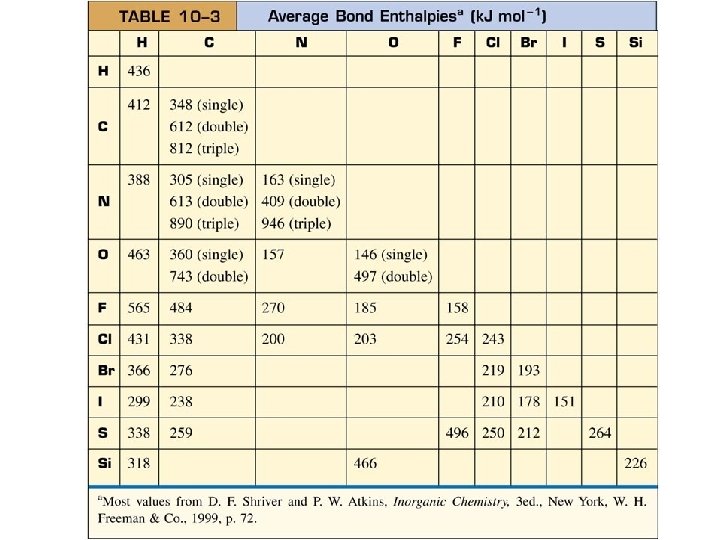
BOND ENTHALPIES • Energy used to BREAK Specific Bond in Gas Phase Reaction • N. B. Bond Enthalpies are ALWAYS +.
