Stabilization of Catalytic Surfaces using Bimetallic CoreShell Structures

Stabilization of Catalytic Surfaces using Bimetallic Core‐Shell Structures Andrew P. Wong 1, John Tengco 1, Arthur Reber 2, Stavros Karakalos 1, Shiv Khanna 2, John R. Regalbuto 1, and John R. Monnier 1 1 Department of Chemical Engineering, USC 2 Department of Physics, VCU June 7 th, 2017 25 th North American Catalysis Society Meeting Denver, CO
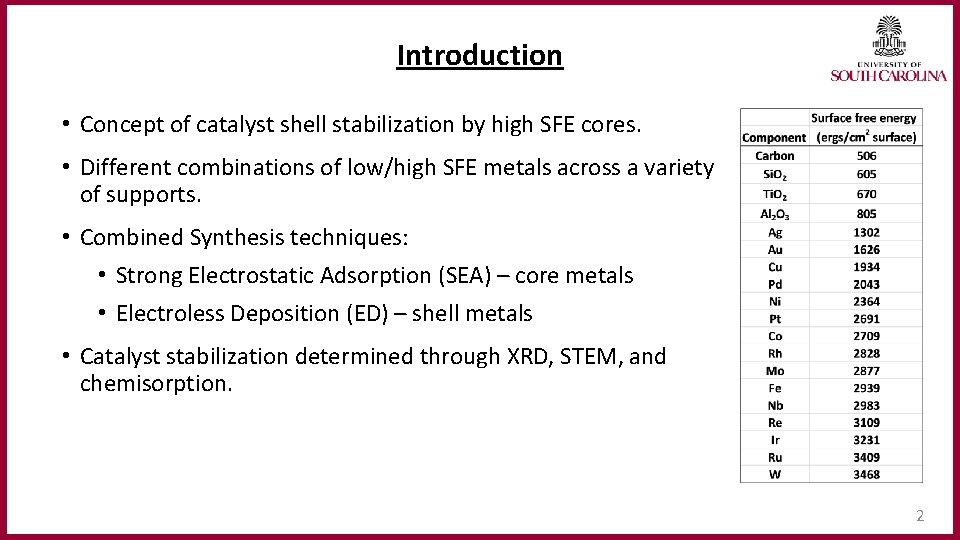
Introduction • Concept of catalyst shell stabilization by high SFE cores. • Different combinations of low/high SFE metals across a variety of supports. • Combined Synthesis techniques: • Strong Electrostatic Adsorption (SEA) – core metals • Electroless Deposition (ED) – shell metals • Catalyst stabilization determined through XRD, STEM, and chemisorption. 2
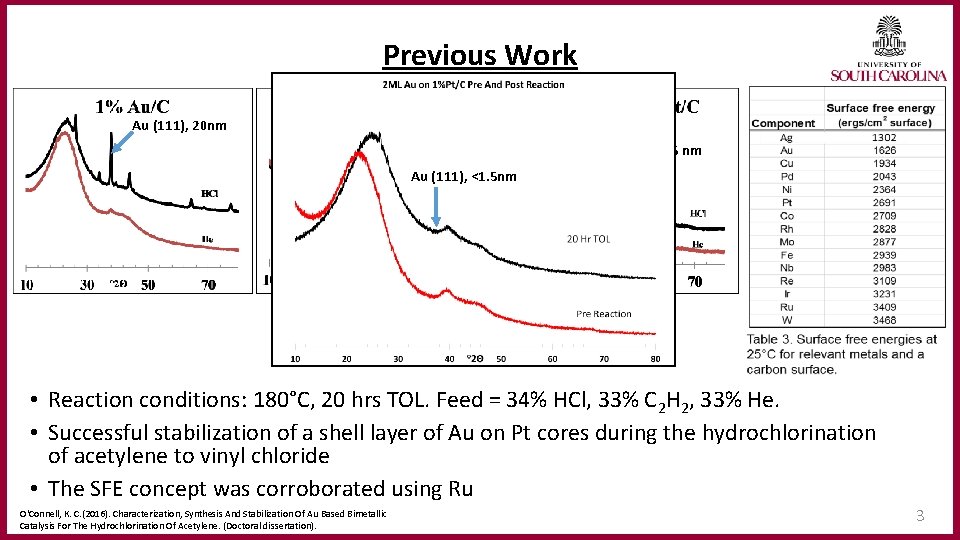
Previous Work Au (111), 20 nm Pt (111), 1. 7 nm Au (111), <1. 5 nm • Reaction conditions: 180°C, 20 hrs TOL. Feed = 34% HCl, 33% C 2 H 2, 33% He. • Successful stabilization of a shell layer of Au on Pt cores during the hydrochlorination of acetylene to vinyl chloride • The SFE concept was corroborated using Ru O'Connell, K. C. (2016). Characterization, Synthesis And Stabilization Of Au Based Bimetallic Catalysis For The Hydrochlorination Of Acetylene. (Doctoral dissertation). 3
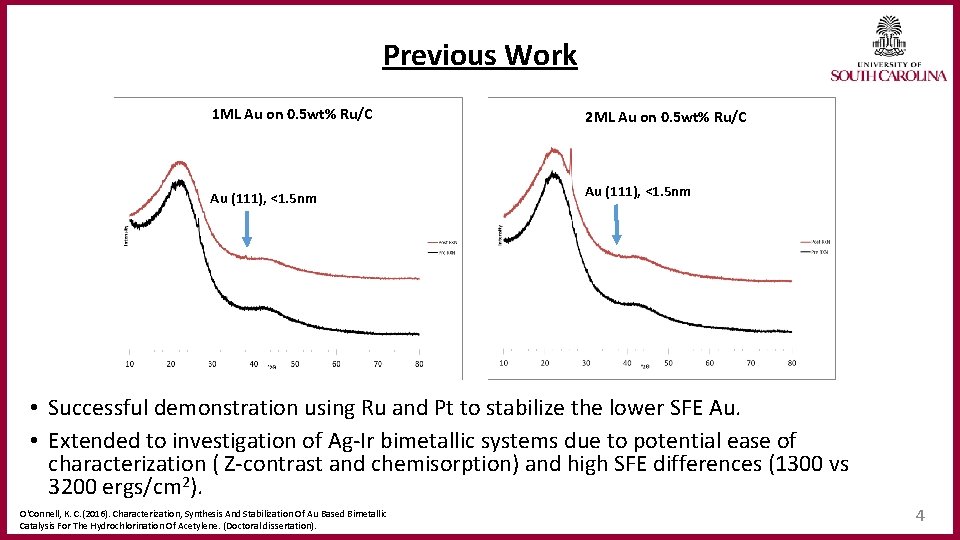
Previous Work 1 ML Au on 0. 5 wt% Ru/C 2 ML Au on 0. 5 wt% Ru/C Au (111), <1. 5 nm • Successful demonstration using Ru and Pt to stabilize the lower SFE Au. • Extended to investigation of Ag-Ir bimetallic systems due to potential ease of characterization ( Z-contrast and chemisorption) and high SFE differences (1300 vs 3200 ergs/cm 2). O'Connell, K. C. (2016). Characterization, Synthesis And Stabilization Of Au Based Bimetallic Catalysis For The Hydrochlorination Of Acetylene. (Doctoral dissertation). 4

Strong Electrostatic Adsorption p. H @ PZC p. H < PZC O- support p. H > PZC Ir[Cl(NH 3)5]2+ cationic complex [Ir. Cl 6]2 -/Al 2 O 3 Ir[Cl(NH 3)5]2+/Si. O 2 OH OH 2+ [Ir. Cl 6]2 H 2 O [Ir. Cl 6]2 anionic complex ort pp su - resulting close packed monolayer of ionic complex (retaining hydration sheaths) with strong interaction with support reduction treatment Δ + H 2 Ir 0 ort p sup - decreased mobility of metal atoms result in smaller catalyst particles (compared to simple impregnation) 5
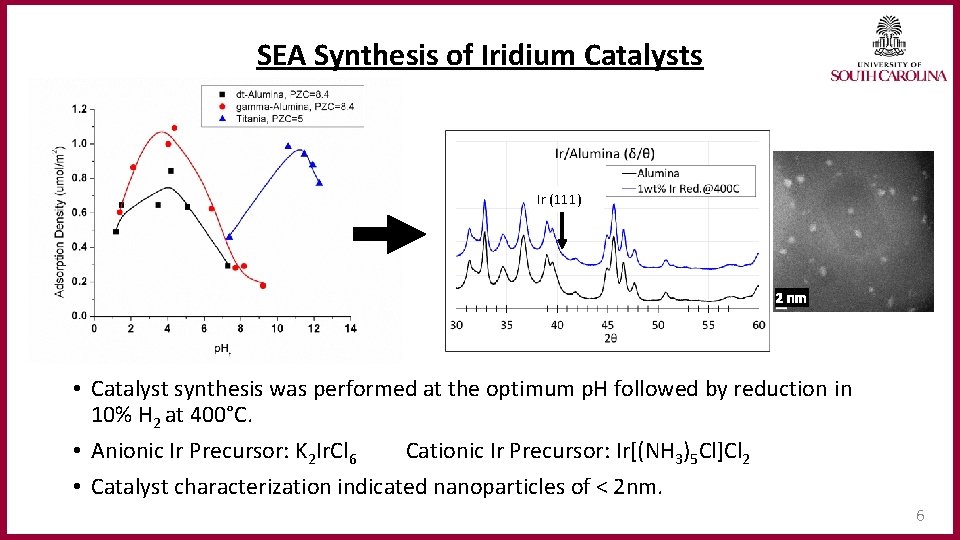
SEA Synthesis of Iridium Catalysts Ir (111) 2 nm • Catalyst synthesis was performed at the optimum p. H followed by reduction in 10% H 2 at 400°C. • Anionic Ir Precursor: K 2 Ir. Cl 6 Cationic Ir Precursor: Ir[(NH 3)5 Cl]Cl 2 • Catalyst characterization indicated nanoparticles of < 2 nm. 6
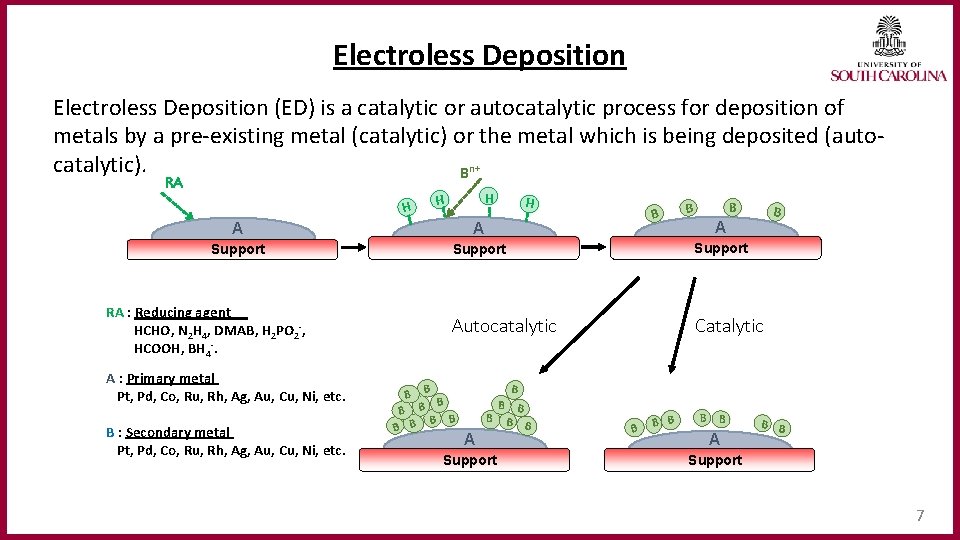
Electroless Deposition (ED) is a catalytic or autocatalytic process for deposition of metals by a pre-existing metal (catalytic) or the metal which is being deposited (autocatalytic). Bn+ RA H H B B A A A Support Autocatalytic Catalytic RA : Reducing agent HCHO, N 2 H 4, DMAB, H 2 PO 2‐, HCOOH, BH 4‐. A : Primary metal Pt, Pd, Co, Ru, Rh, Ag, Au, Cu, Ni, etc. B : Secondary metal Pt, Pd, Co, Ru, Rh, Ag, Au, Cu, Ni, etc. B B B A Support B B B B B A B B Support 7
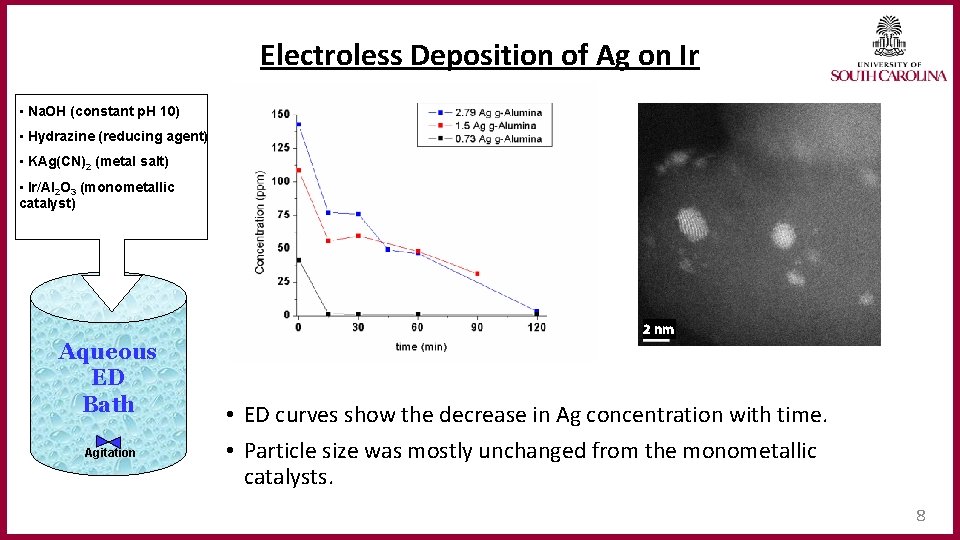
Electroless Deposition of Ag on Ir • Na. OH (constant p. H 10) • Hydrazine (reducing agent) • KAg(CN)2 (metal salt) • Ir/Al 2 O 3 (monometallic catalyst) 2 nm Aqueous ED Bath Agitation • ED curves show the decrease in Ag concentration with time. • Particle size was mostly unchanged from the monometallic catalysts. 8
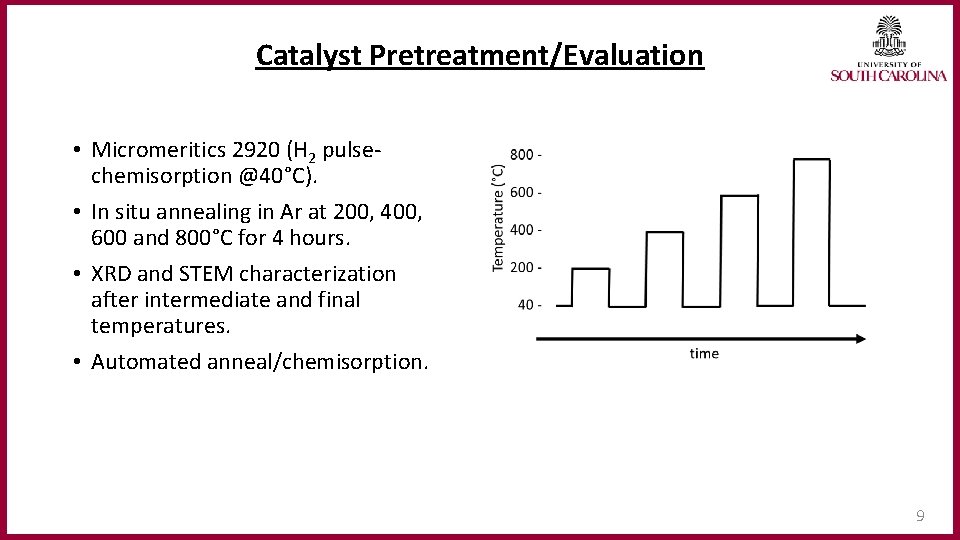
Catalyst Pretreatment/Evaluation • Micromeritics 2920 (H 2 pulsechemisorption @40°C). • In situ annealing in Ar at 200, 400, 600 and 800°C for 4 hours. • XRD and STEM characterization after intermediate and final temperatures. • Automated anneal/chemisorption. 9
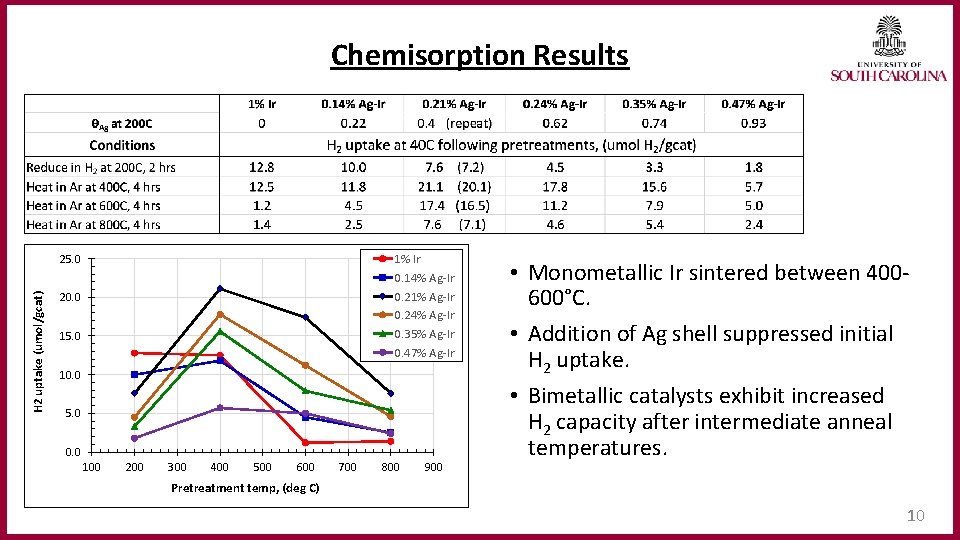
Chemisorption Results 1% Ir 0. 14% Ag-Ir 0. 21% Ag-Ir 0. 24% Ag-Ir 0. 35% Ag-Ir 0. 47% Ag-Ir H 2 uptake (umol/gcat) 25. 0 20. 0 15. 0 10. 0 5. 0 0. 0 100 200 300 400 500 600 700 800 900 • Monometallic Ir sintered between 400600°C. • Addition of Ag shell suppressed initial H 2 uptake. • Bimetallic catalysts exhibit increased H 2 capacity after intermediate anneal temperatures. Pretreatment temp, (deg C) 10
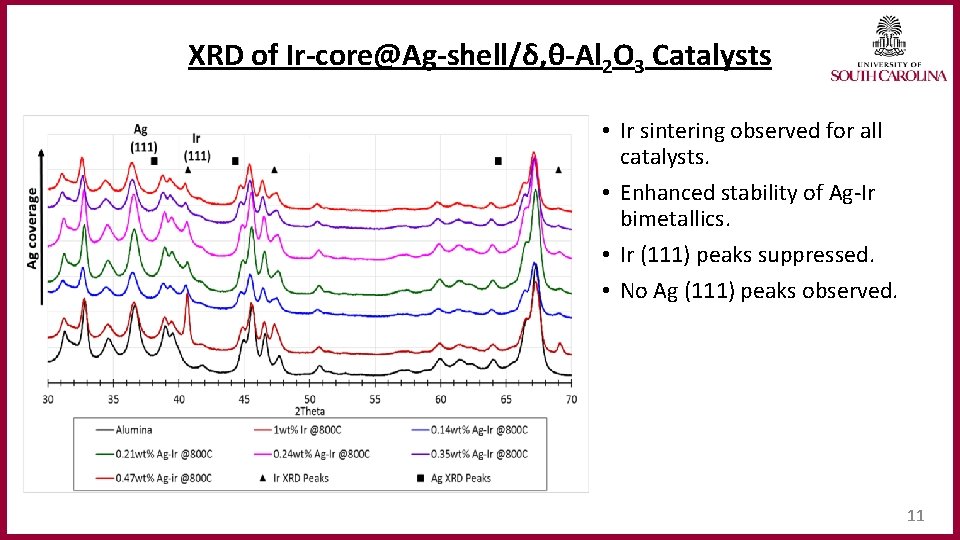
XRD of Ir‐core@Ag‐shell/δ, θ‐Al 2 O 3 Catalysts • Ir sintering observed for all catalysts. • Enhanced stability of Ag-Ir bimetallics. • Ir (111) peaks suppressed. • No Ag (111) peaks observed. 11
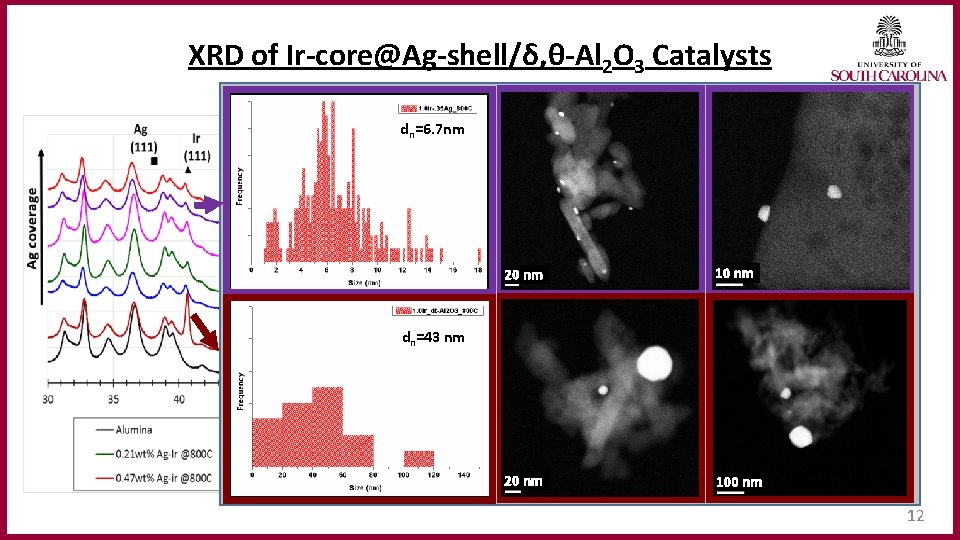
XRD of Ir‐core@Ag‐shell/δ, θ‐Al 2 O 3 Catalysts dn=6. 7 nm 20 nm • Ir sintering observed for all catalysts. • Enhanced stability of Ag-Ir bimetallics. • Ir (111) peaks suppressed. 10 nm • No Ag (111) peaks observed. dn=43 nm 20 nm 100 nm 12
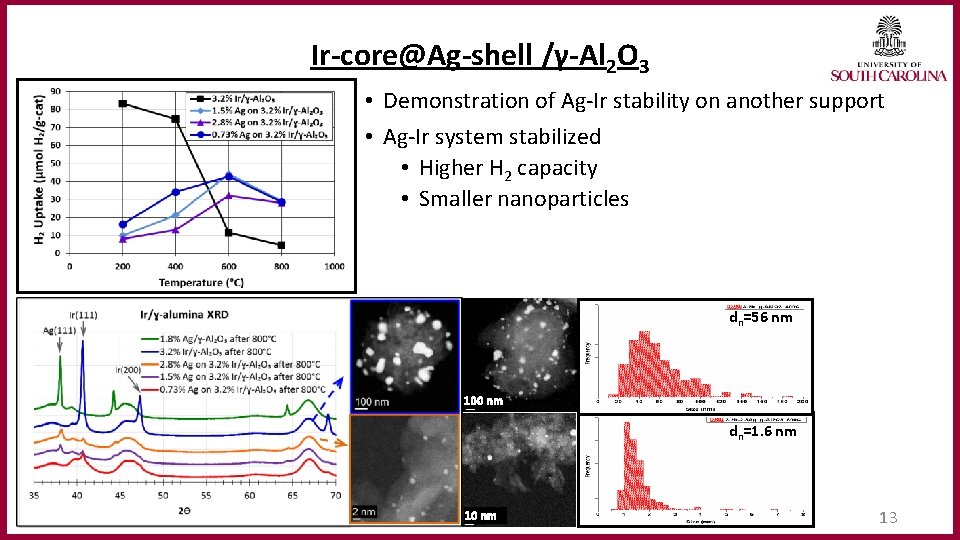
Ir‐core@Ag‐shell /γ‐Al 2 O 3 • Demonstration of Ag-Ir stability on another support • Ag-Ir system stabilized • Higher H 2 capacity • Smaller nanoparticles dn=56 nm 100 nm dn=1. 6 nm 10 nm 13
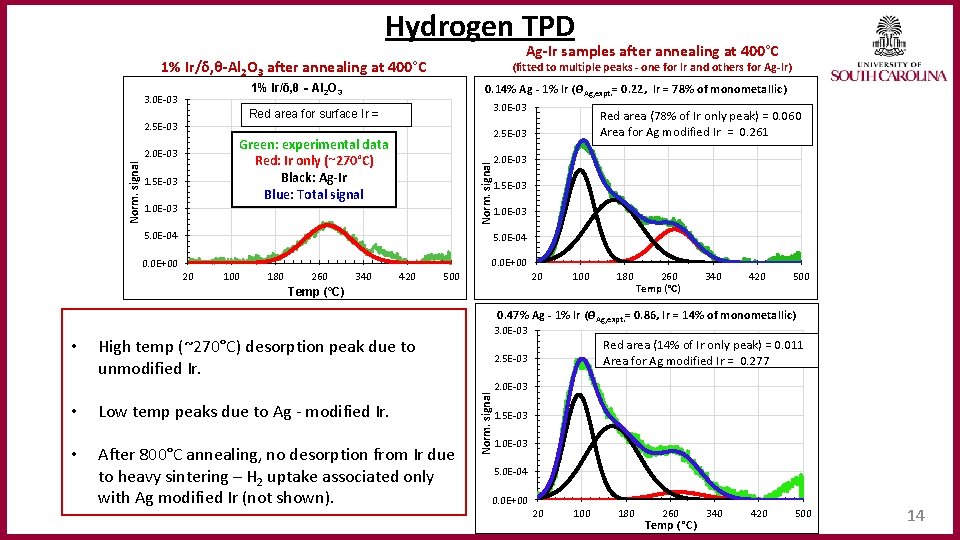
Hydrogen TPD Ag‐Ir samples after annealing at 400°C 1% Ir/δ, θ‐Al 2 O 3 after annealing at 400°C 0. 14% Ag ‐ 1% Ir (ϴAg, expt. = 0. 22, Ir = 78% of monometallic) 1% Ir/δ, θ‐Al 2 O 3 3. 0 E-03 Red area for surface Ir = 2. 5 E-03 1. 0 E-03 2. 0 E-03 Norm. signal 1. 5 E-03 Red area (78% of Ir only peak) = 0. 060 Area for Ag modified Ir = 0. 261 2. 5 E-03 Green: experimental data Red: Ir only (~270°C) Black: Ag‐Ir Blue: Total signal 2. 0 E-03 Norm. signal (fitted to multiple peaks ‐ one for Ir and others for Ag‐Ir) 1. 5 E-03 1. 0 E-03 5. 0 E-04 0. 0 E+00 20 100 180 260 340 420 20 500 180 Temp (°C) 260 Temp (°C) 340 420 500 0. 47% Ag ‐ 1% Ir (ϴAg, expt. = 0. 86, Ir = 14% of monometallic) • High temp (~270°C) desorption peak due to unmodified Ir. 3. 0 E-03 Red area (14% of Ir only peak) = 0. 011 Area for Ag modified Ir = 0. 277 2. 5 E-03 • • Low temp peaks due to Ag - modified Ir. After 800°C annealing, no desorption from Ir due to heavy sintering – H 2 uptake associated only with Ag modified Ir (not shown). Norm. signal 2. 0 E-03 1. 5 E-03 1. 0 E-03 5. 0 E-04 0. 0 E+00 20 100 180 260 Temp ( °C) 340 420 500 14
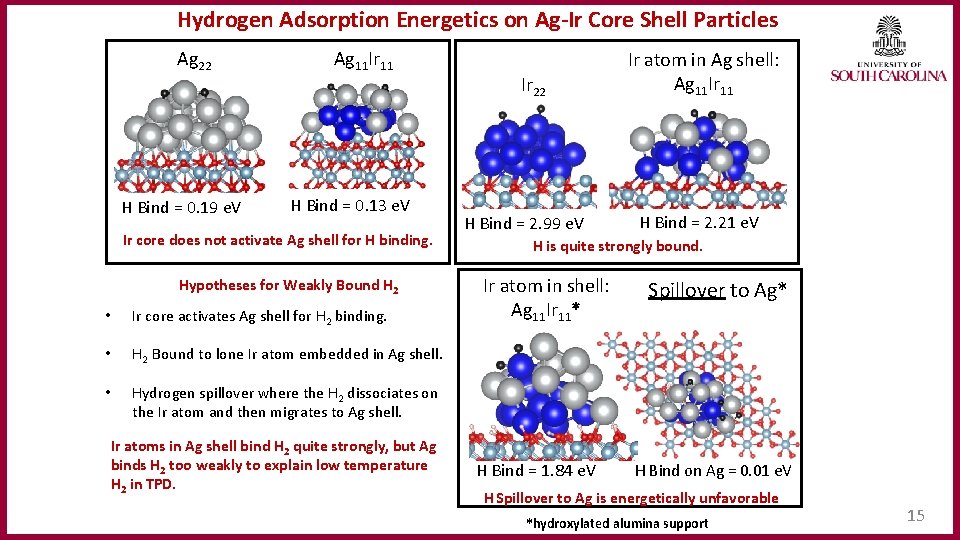
Hydrogen Adsorption Energetics on Ag‐Ir Core Shell Particles Ag 22 H Bind = 0. 19 e. V Ag 11 Ir 11 H Bind = 0. 13 e. V Ir core does not activate Ag shell for H binding. Hypotheses for Weakly Bound H 2 • Ir core activates Ag shell for H 2 binding. • H 2 Bound to lone Ir atom embedded in Ag shell. • Hydrogen spillover where the H 2 dissociates on the Ir atom and then migrates to Ag shell. Ir atoms in Ag shell bind H 2 quite strongly, but Ag binds H 2 too weakly to explain low temperature H 2 in TPD. Ir 22 H Bind = 2. 99 e. V Ir atom in Ag shell: Ag 11 Ir 11 H Bind = 2. 21 e. V H is quite strongly bound. Ir atom in shell: Ag 11 Ir 11* H Bind = 1. 84 e. V Spillover to Ag* H Bind on Ag = 0. 01 e. V H Spillover to Ag is energetically unfavorable *hydroxylated alumina support 15
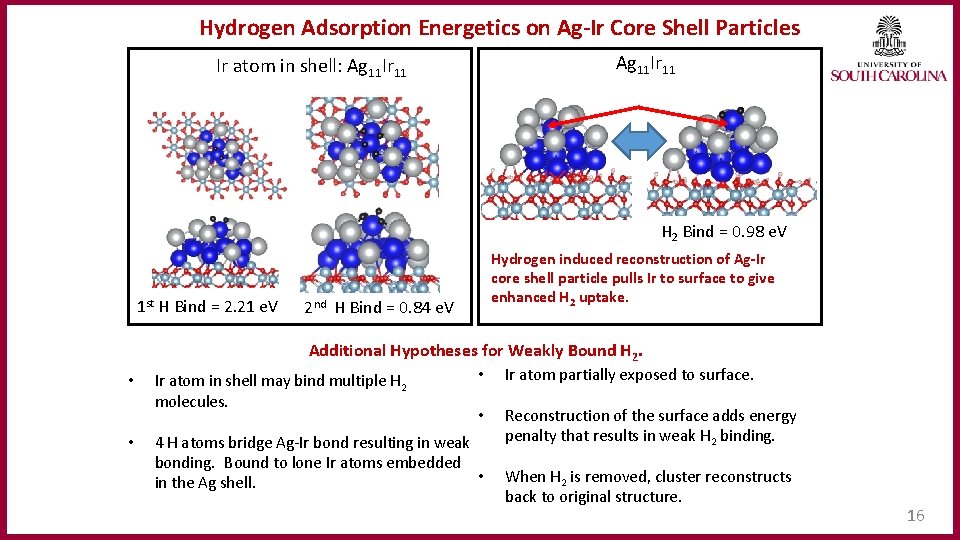
Hydrogen Adsorption Energetics on Ag‐Ir Core Shell Particles Ag 11 Ir 11 Ir atom in shell: Ag 11 Ir 11 H 2 Bind = 0. 98 e. V 1 st H Bind = 2. 21 e. V • 2 nd H Bind = 0. 84 e. V Additional Hypotheses for Weakly Bound H 2. • Ir atom partially exposed to surface. Ir atom in shell may bind multiple H molecules. • Hydrogen induced reconstruction of Ag‐Ir core shell particle pulls Ir to surface to give enhanced H 2 uptake. 2 • 4 H atoms bridge Ag-Ir bond resulting in weak bonding. Bound to lone Ir atoms embedded • in the Ag shell. Reconstruction of the surface adds energy penalty that results in weak H 2 binding. When H 2 is removed, cluster reconstructs back to original structure. 16
Summary • Combination of Strong Electrostatic Adsorption and Electroless Deposition (Core@Shell). • Demonstrated shell stabilization by high SFE cores (Au-Pt, Au-Ru, Ag-Ir). • Bimetallics retained higher H 2 capacity/smaller particles after annealing. • TPD showed new hydrogen adsorption species. • Computational studies suggested H/Ir stoichiometry is dependent on Ir size/structure. 17
Acknowledgements • The Center of Rational Catalyst Synthesis (NSF I/UCRC Ce. RCa. S) • My colleagues at the University of South Carolina (Dr. John Tengco and Sonia Eskandari) • Virginia Commonwealth University 18

Thank you!!! Questions? 19
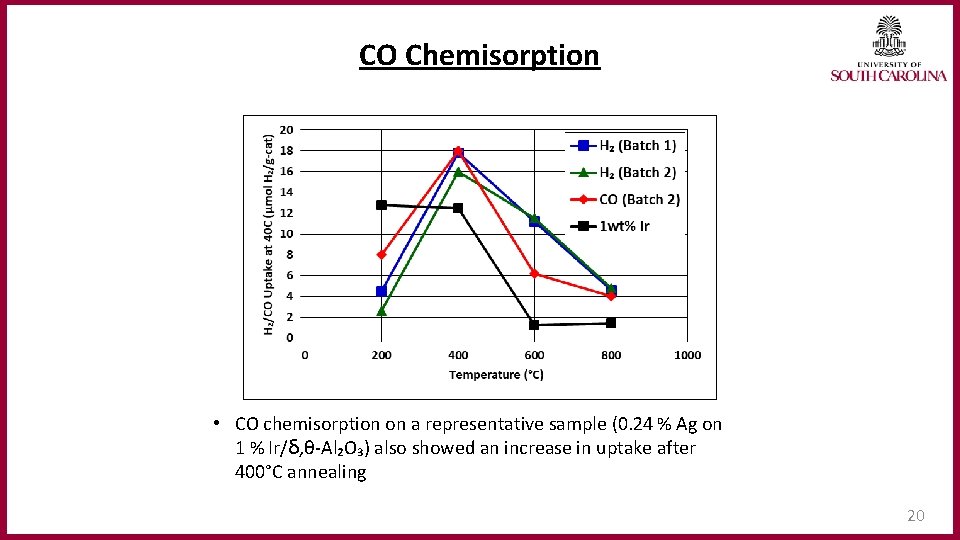
CO Chemisorption • CO chemisorption on a representative sample (0. 24 % Ag on 1 % Ir/ẟ, θ-Al₂O₃) also showed an increase in uptake after 400°C annealing 20
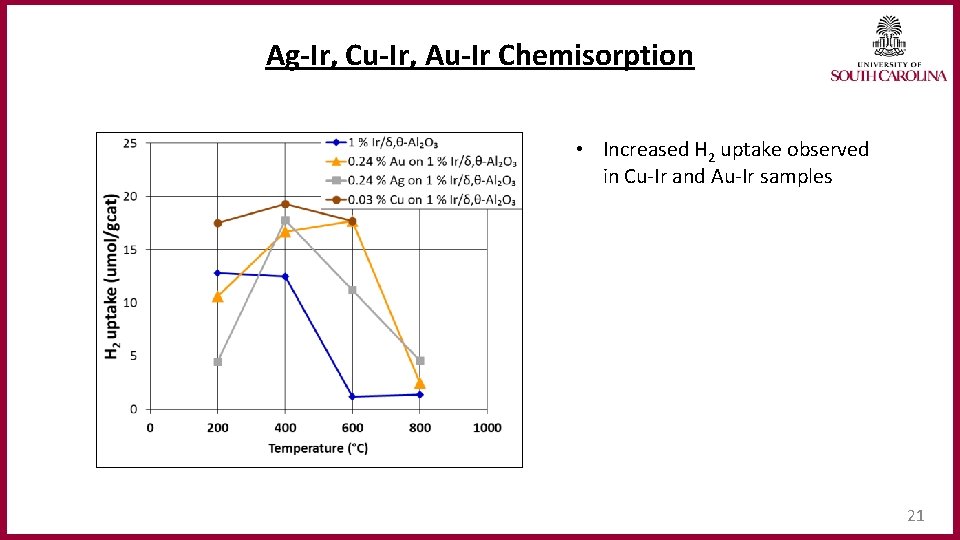
Ag‐Ir, Cu‐Ir, Au‐Ir Chemisorption • Increased H 2 uptake observed in Cu-Ir and Au-Ir samples 21
- Slides: 21