Stability 1 What is stability USP Definition Extent
Stability 1

What is stability? (USP Definition) Extent to which product retains, within specified limits…the same properties and characteristics that it possessed at manufacture. Chemical, Physical, Microbiological, Therapeutic, Toxicological What is stability? (ICH Definition) …how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental factors such as temperature, humidity, and light. 2
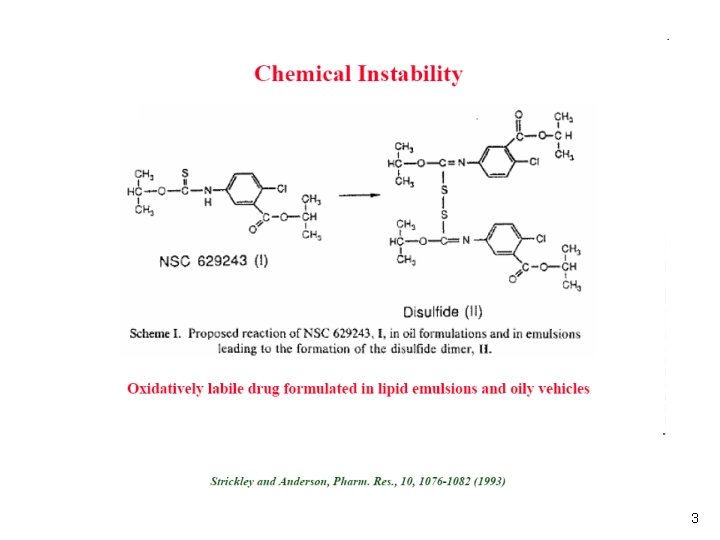
3

4
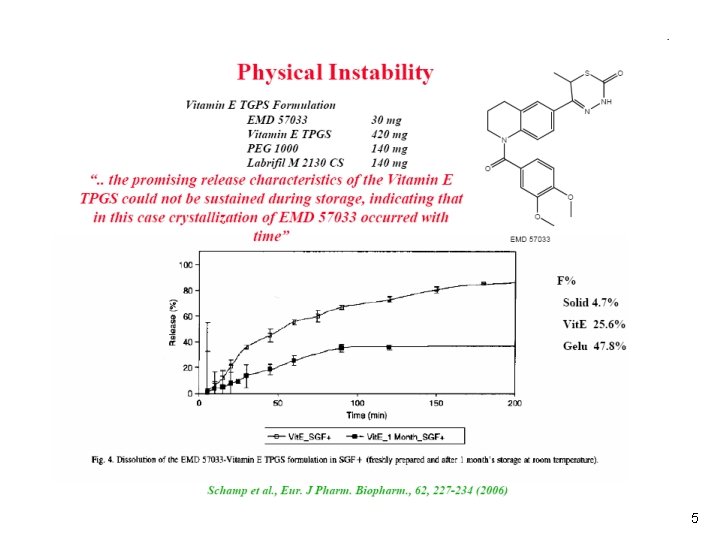
5

Stability Studies Stability Testing The purpose of stability testing is to study and document the effect of environmental factors such as temperature, humidity and light on the physical and chemical quality of a drug product and to established a shelf-life and recommended storage conditions based on the effect of these environmental factors on the drug product over time. 6

Factors effecting product stability Temperature, light, air, humidity and package components Batch to batch variation (particle size, hardness, initial content) Must gain an intimate knowledge of product stability to be successful Why do stability testing? Find most stable dosage form Determine the most appropriate packaging and labeling Determine clinical retest periods and final drug product expiry periods Define the storage and transport conditions Plus. . . It’s Just a Lot of Darn Fun 7

Global Primary Stability Program Goal: Determine shelf life in the selected packages for the climate zones the product will be marketed in Three batches of pilot or above (If Full-scale production, be a hero and make it a commitment batch also!!!!) Protocol must meet requirements spelled out in ICH Guidelines (Q 1 A, Q 1 B International conference on harmonisation USA, EU, J) – 25/60 long term (for life of product (2 yr? ) - statistical analysis) – 40/75 accelerated (3, 6 months - degradation info. , 4 x rule) – 30/60 Intermediate (3, 6, 9, 12 - backup if 40/75 has significant change 2 x rule) WHO and other non ICH conditions – 30/70 long term for climatic zones 3 and 4 (statistical analysis) – 30/40 drug product in semi-permeable container 8

ICH Storage Conditions for Formal Stability Studies ICH General Storage Requirements Study Storage Condition Minimum time period covered by data at submission 25 C ± 2 C / 60%RH ± 5%RH Long term* 30 C ± 2 C / 65%RH ± 5%RH 12 months Intermediate** 30 C ± 2 C / 65%RH ± 5%RH 6 months Accelerated 40 C ± 2 C / 75%RH ± 5%RH 6 months 9
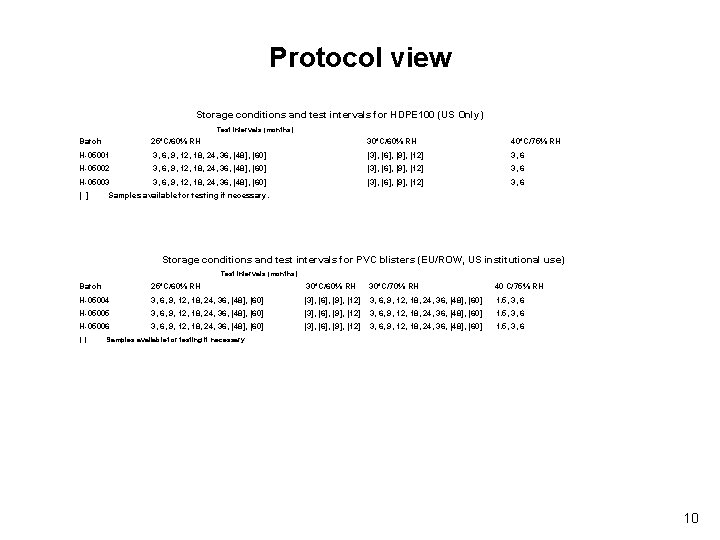
Protocol view Storage conditions and test intervals for HDPE 100 (US Only) Test intervals (months) Batch 25°C/60% RH 30°C/60% RH 40°C/75% RH H-05001 3, 6, 9, 12, 18, 24, 36, [48], [60] [3], [6], [9], [12] 3, 6 H-05002 3, 6, 9, 12, 18, 24, 36, [48], [60] [3], [6], [9], [12] 3, 6 H-05003 3, 6, 9, 12, 18, 24, 36, [48], [60] [3], [6], [9], [12] 3, 6 [ ] Samples available for testing if necessary. Storage conditions and test intervals for PVC blisters (EU/ROW, US institutional use) Test intervals (months) Batch 25°C/60% RH 30°C/70% RH 40 C/75% RH H-05004 3, 6, 9, 12, 18, 24, 36, [48], [60] [3], [6], [9], [12] 3, 6, 9, 12, 18, 24, 36, [48], [60] 1. 5, 3, 6 H-05005 3, 6, 9, 12, 18, 24, 36, [48], [60] [3], [6], [9], [12] 3, 6, 9, 12, 18, 24, 36, [48], [60] 1. 5, 3, 6 H-05006 3, 6, 9, 12, 18, 24, 36, [48], [60] [3], [6], [9], [12] 3, 6, 9, 12, 18, 24, 36, [48], [60] 1. 5, 3, 6 [ ] Samples available for testing if necessary. 10

Stability report l Summary - shelf life l Information on program - batches, etc. l tests performed l results and discussions l PROTOCOL DEVIATIONS!!!! l Results table l Statistical evaluations and graphs (if nec. ) l Different versions for different regions of the world if different information is required 11
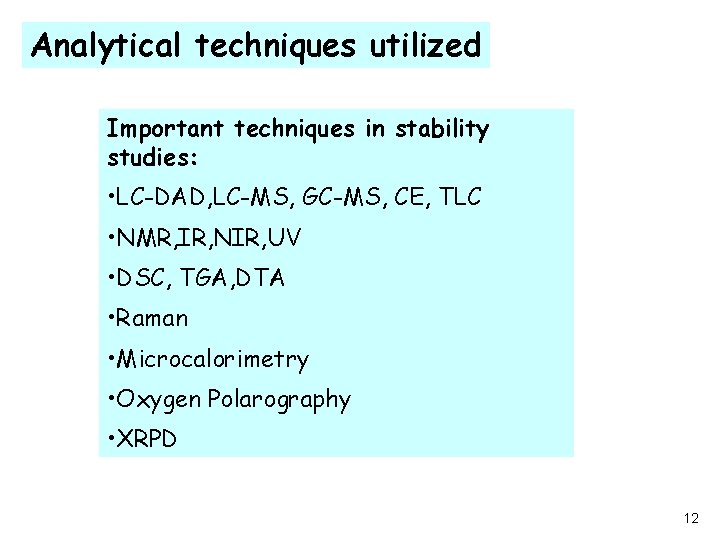
Analytical techniques utilized Important techniques in stability studies: • LC-DAD, LC-MS, GC-MS, CE, TLC • NMR, IR, NIR, UV • DSC, TGA, DTA • Raman • Microcalorimetry • Oxygen Polarography • XRPD 12

Objectives of preformulation stability studies • Identify degradation pathways • Identify compatible excipient(s) • Identify stabilizers • Specify rational formulation development. • Minimize the formulation variants tested. 13
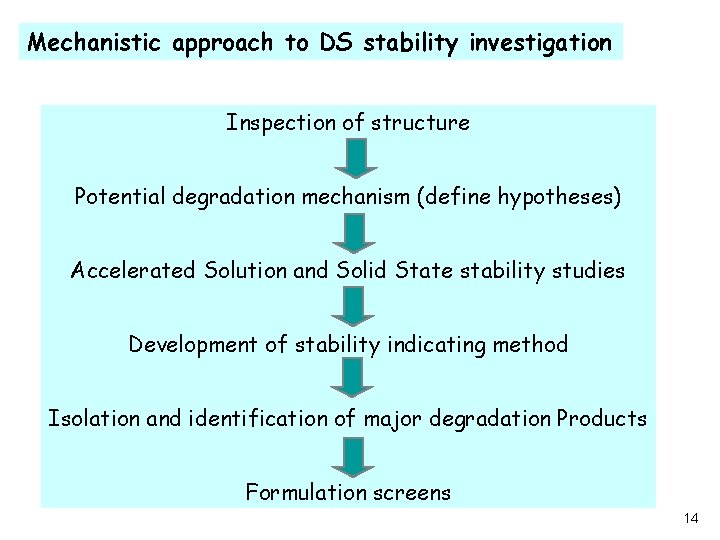
Mechanistic approach to DS stability investigation Inspection of structure Potential degradation mechanism (define hypotheses) Accelerated Solution and Solid State stability studies Development of stability indicating method Isolation and identification of major degradation Products Formulation screens 14
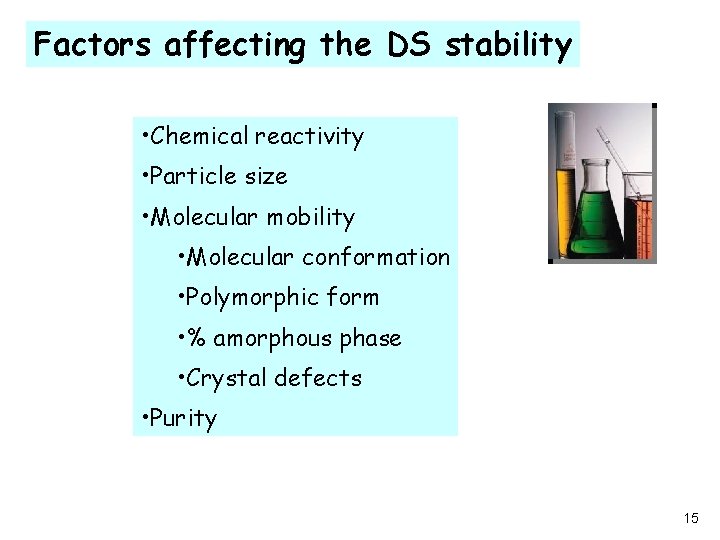
Factors affecting the DS stability • Chemical reactivity • Particle size • Molecular mobility • Molecular conformation • Polymorphic form • % amorphous phase • Crystal defects • Purity 15
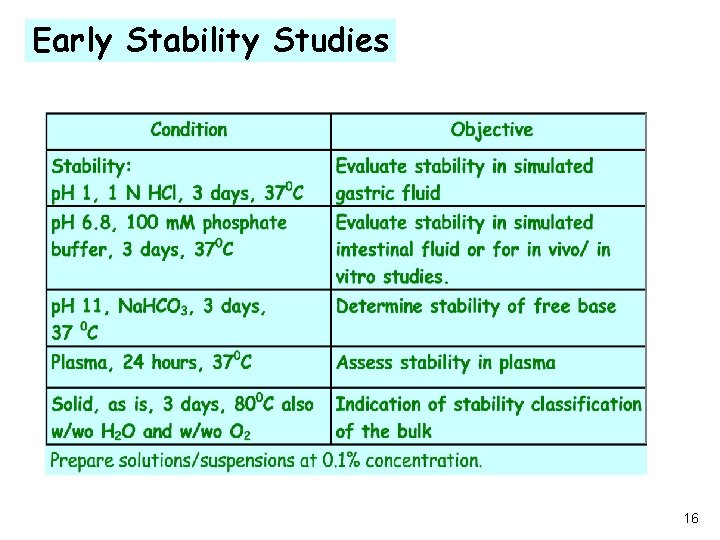
Early Stability Studies 16
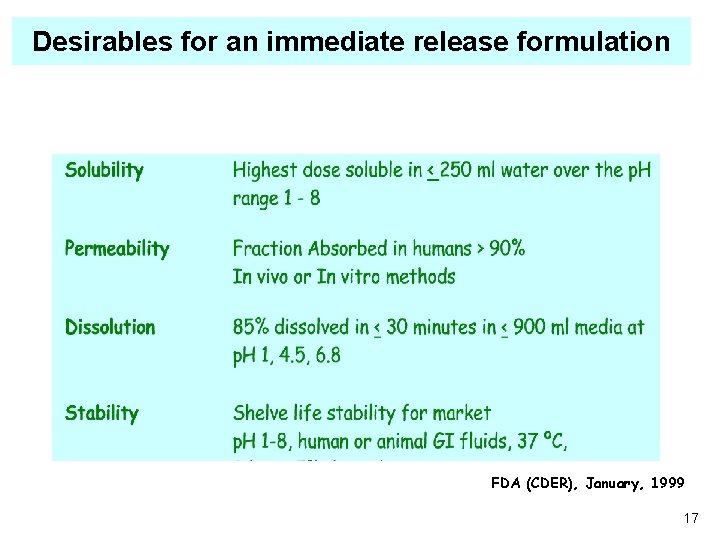
Desirables for an immediate release formulation FDA (CDER), January, 1999 17
Stability Case study I Molecular mobility Ace inhibitor TI 211 -950 SPIRAPRIL Courtesy: R. Vivilecchia, B. Ross 18
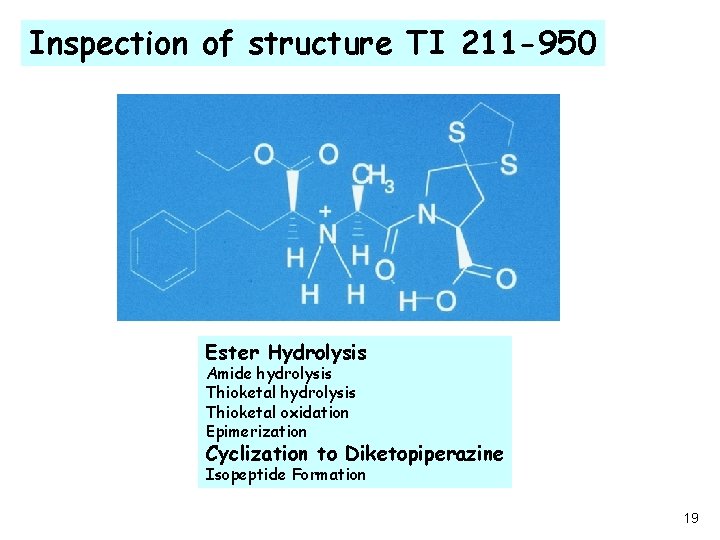
Inspection of structure TI 211 -950 Ester Hydrolysis Amide hydrolysis Thioketal oxidation Epimerization Cyclization to Diketopiperazine Isopeptide Formation 19
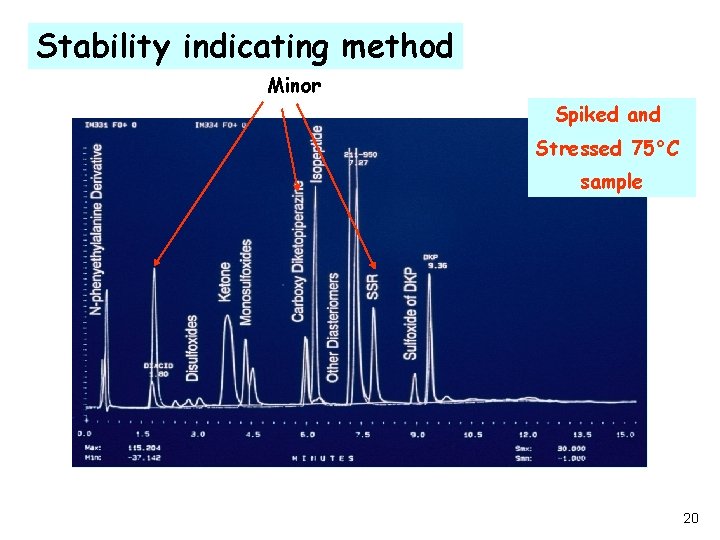
Stability indicating method Minor Spiked and Stressed 75°C sample 20
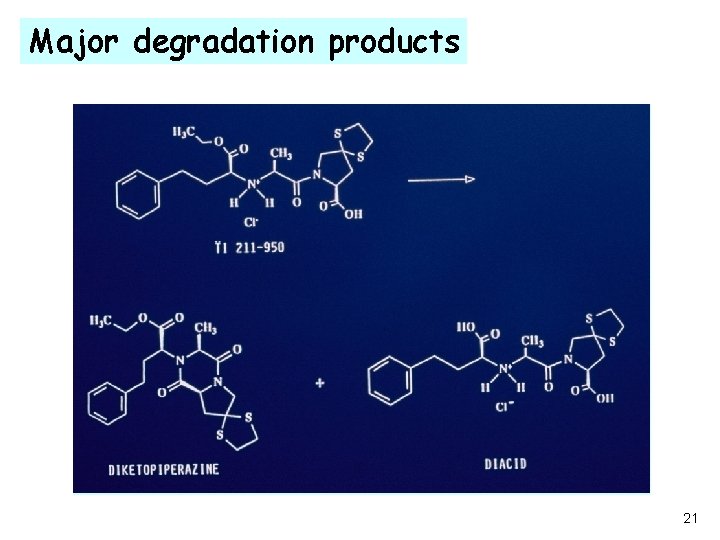
Major degradation products 21

Challenge ·The major degradation products of TI 211 -950 are; - the di-acid hydrolysis product, an active metabolite and, -the diketopiperazine dehydration product, major concern. ·The presence of water accelerates the formation rate of diketopiperazine. A dehydration reaction accelerated by water is unusual!!!!! ·Since water is present in all pharmaceutical excipients and environment, there was a critical need to elucidate the reaction mechanism. ·Rapid screens, under stress conditions, to find a viable and stable formulation. 22
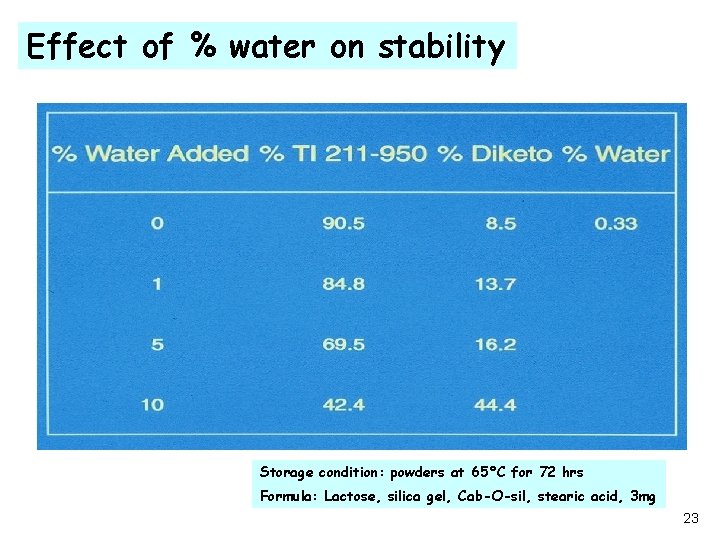
Effect of % water on stability Storage condition: powders at 65°C for 72 hrs Formula: Lactose, silica gel, Cab-O-sil, stearic acid, 3 mg 23
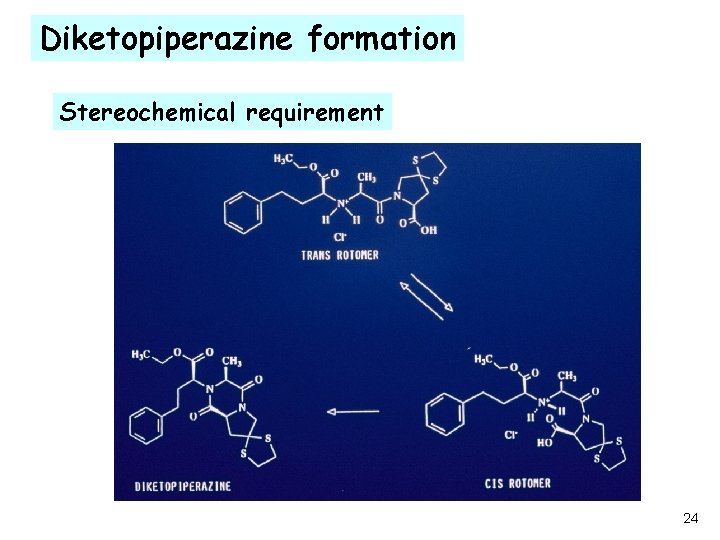
Diketopiperazine formation Stereochemical requirement 24
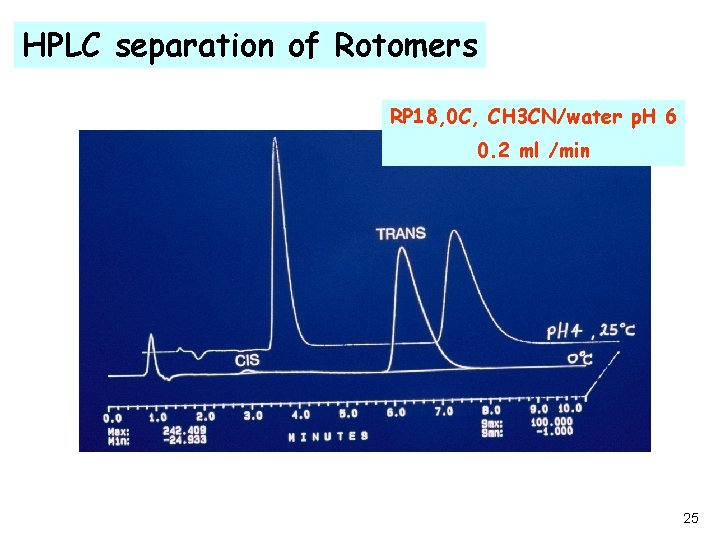
HPLC separation of Rotomers RP 18, 0 C, CH 3 CN/water p. H 6 0. 2 ml /min 25
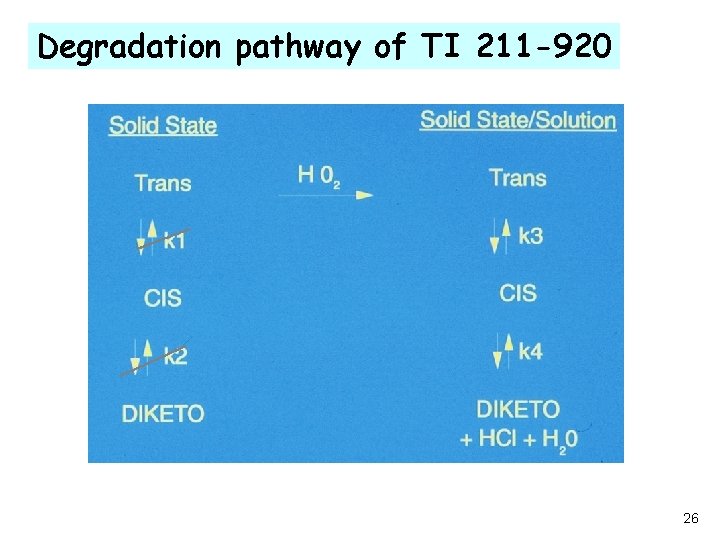
Degradation pathway of TI 211 -920 26
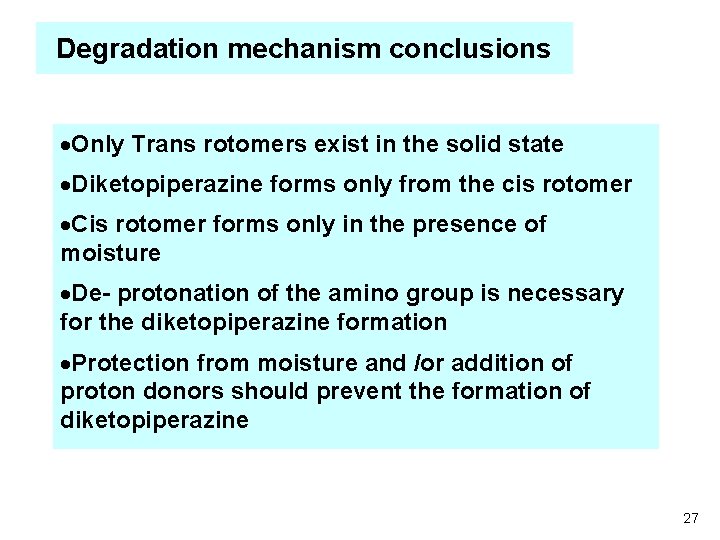
Degradation mechanism conclusions ·Only Trans rotomers exist in the solid state ·Diketopiperazine forms only from the cis rotomer ·Cis rotomer forms only in the presence of moisture ·De- protonation of the amino group is necessary for the diketopiperazine formation ·Protection from moisture and /or addition of proton donors should prevent the formation of diketopiperazine 27
Stability Case Study II Chirality and Patents 28
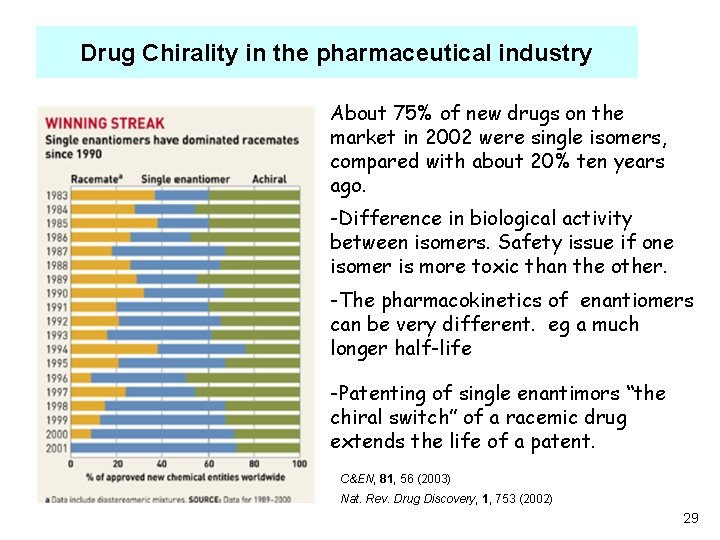
Drug Chirality in the pharmaceutical industry About 75% of new drugs on the market in 2002 were single isomers, compared with about 20% ten years ago. -Difference in biological activity between isomers. Safety issue if one isomer is more toxic than the other. -The pharmacokinetics of enantiomers can be very different. eg a much longer half-life -Patenting of single enantimors “the chiral switch” of a racemic drug extends the life of a patent. C&EN, 81, 56 (2003) Nat. Rev. Drug Discovery, 1, 753 (2002) 29
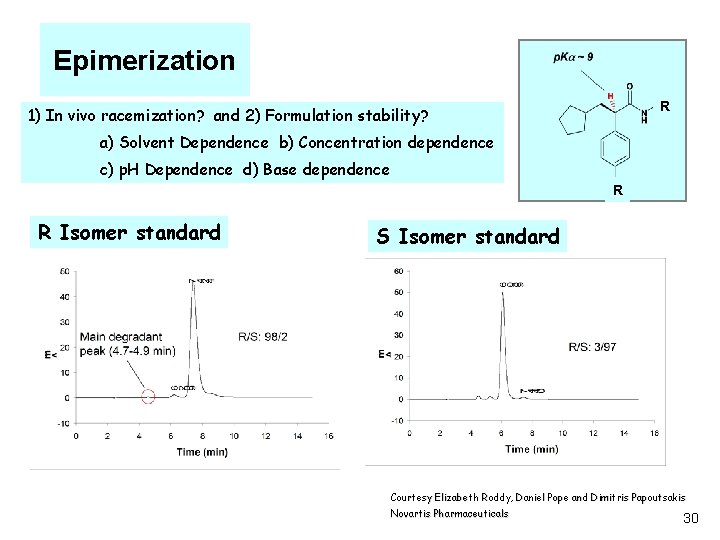
Epimerization R 1) In vivo racemization? and 2) Formulation stability? a) Solvent Dependence b) Concentration dependence c) p. H Dependence d) Base dependence R R Isomer standard S Isomer standard Courtesy Elizabeth Roddy, Daniel Pope and Dimitris Papoutsakis Novartis Pharmaceuticals 30
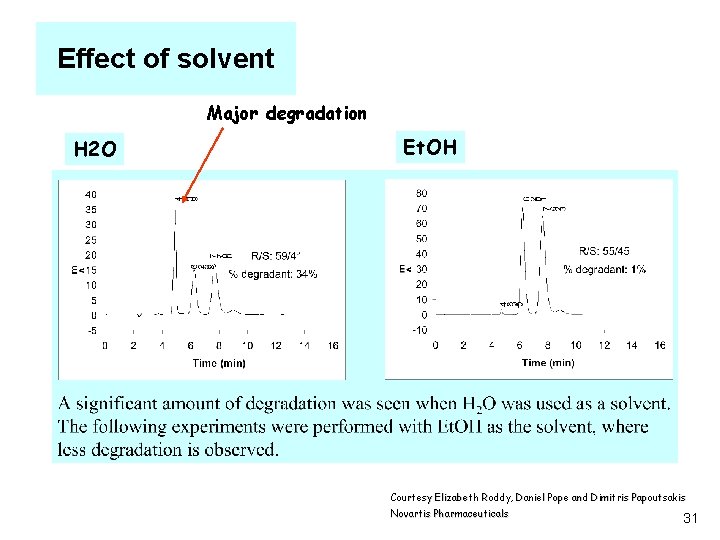
Effect of solvent Major degradation H 2 O Et. OH Courtesy Elizabeth Roddy, Daniel Pope and Dimitris Papoutsakis Novartis Pharmaceuticals 31
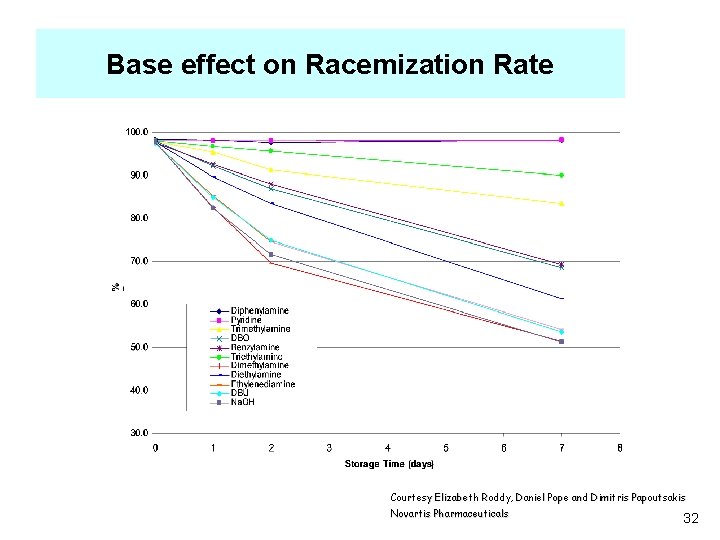
Base effect on Racemization Rate Courtesy Elizabeth Roddy, Daniel Pope and Dimitris Papoutsakis Novartis Pharmaceuticals 32
Conclusions ·Water promotes extensive degradation in addition to racemization; ethanol showed only racemization. ·Kinetics showed a rough p. Ka dependence; weak bases, with p. Ka < 5. 2, did not display racemization, however, ·Base stereochemistry was also a factor affecting the kinetics; i. e. , secondary amines lead to more complete racemization than tertiary amines, and dimethylamine ad more impact relative to triethylamine. ·With very strong bases deprotonated ethanol may act as the base affecting the kinetics; future work will probe the same reactions in aprotic solvents Courtesy Elizabeth Roddy, Daniel Pope and Dimitris Papoutsakis Novartis Pharmaceuticals 33

Stability Case Study III HPLC in DS salt stability 34
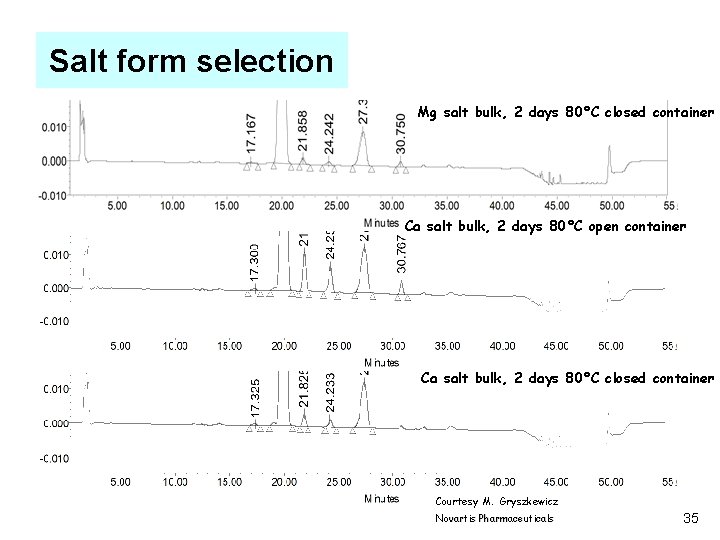
Salt form selection Mg salt bulk, 2 days 80°C closed container Ca salt bulk, 2 days 80°C open container Ca salt bulk, 2 days 80°C closed container Courtesy M. Gryszkewicz Novartis Pharmaceuticals 35

HPLC in Drug-excipient compatibility studies 36
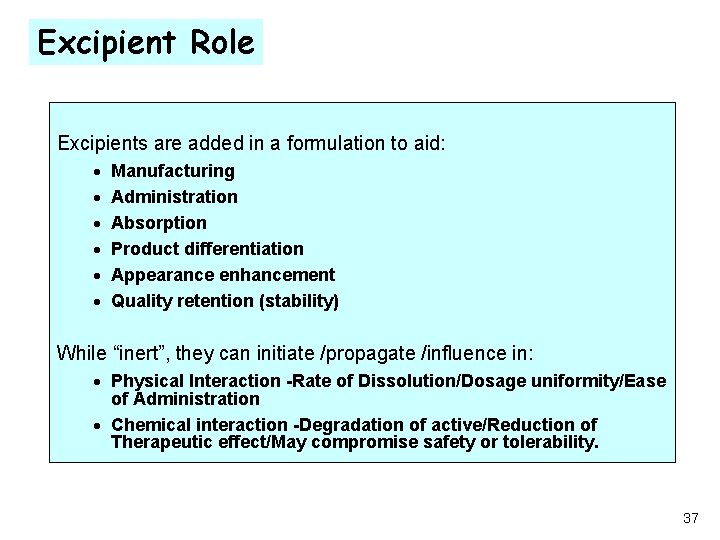
Excipient Role Excipients are added in a formulation to aid: · · · Manufacturing Administration Absorption Product differentiation Appearance enhancement Quality retention (stability) While “inert”, they can initiate /propagate /influence in: · Physical Interaction -Rate of Dissolution/Dosage uniformity/Ease of Administration · Chemical interaction -Degradation of active/Reduction of Therapeutic effect/May compromise safety or tolerability. 37
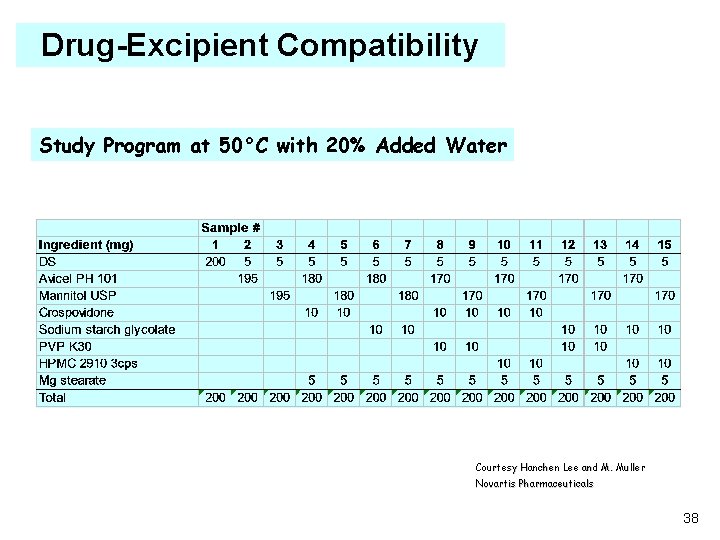
Drug-Excipient Compatibility Study Program at 50°C with 20% Added Water Courtesy Hanchen Lee and M. Muller Novartis Pharmaceuticals 38
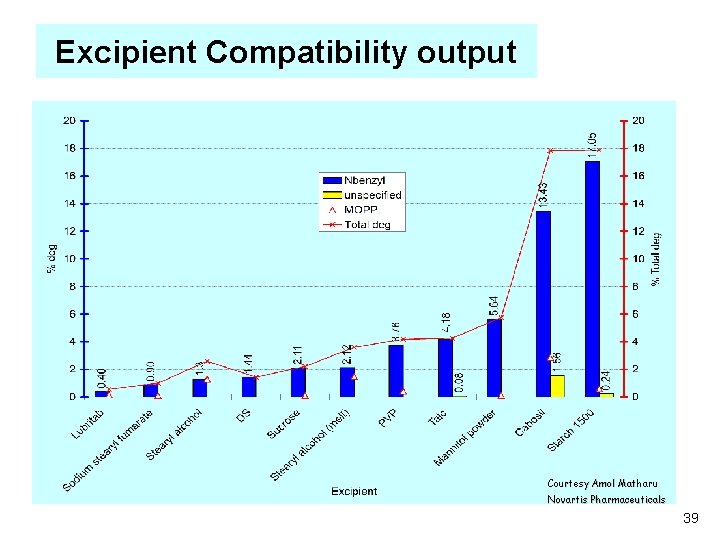
Excipient Compatibility output Courtesy Amol Matharu Novartis Pharmaceuticals 39
Excipient Case study Recovery Challenge Elimination of Metformin-Croscarmelose Interaction by Arginine Competition Courtesy Wei Huang et al Novartis Pharmaceuticals 40
Recovery Challenge • Recovery evaluation experiments during method validation, of a bilayer tablet containing two active DS, indicated large loses of one of these actives, (Metformin). • Investigation of the drug-excipient interaction mechanism was elucidated and recovery improved. 41
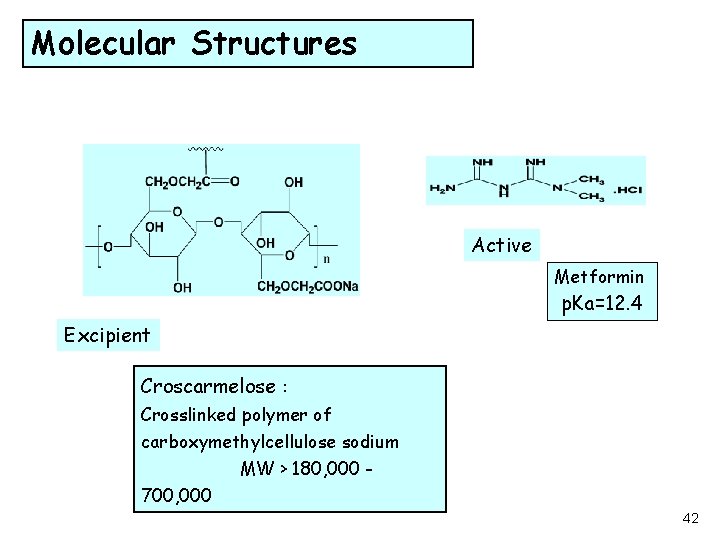
Molecular Structures Active Metformin p. Ka=12. 4 Excipient Croscarmelose : Crosslinked polymer of carboxymethylcellulose sodium MW > 180, 000 700, 000 42
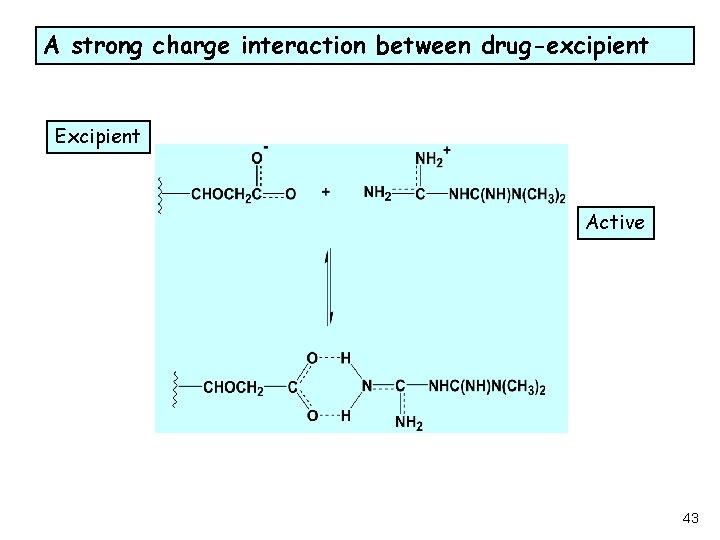
A strong charge interaction between drug-excipient Excipient Active L 43
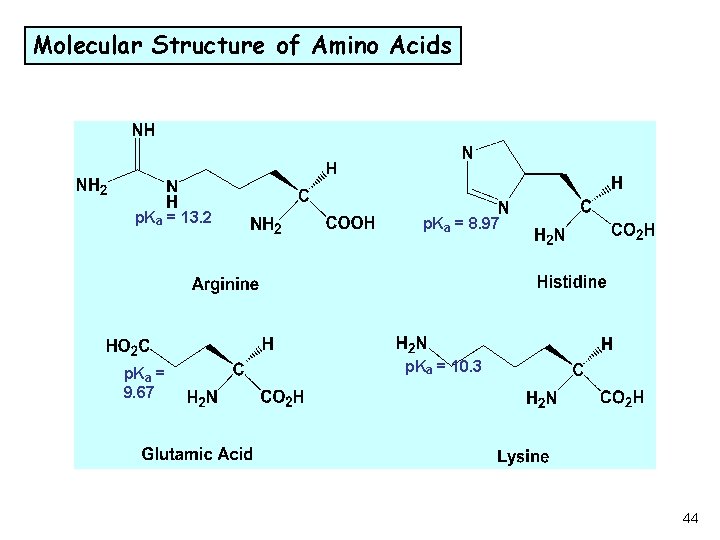
Molecular Structure of Amino Acids p. Ka = 13. 2 p. Ka = 9. 67 p. Ka = 8. 97 p. Ka = 10. 3 44
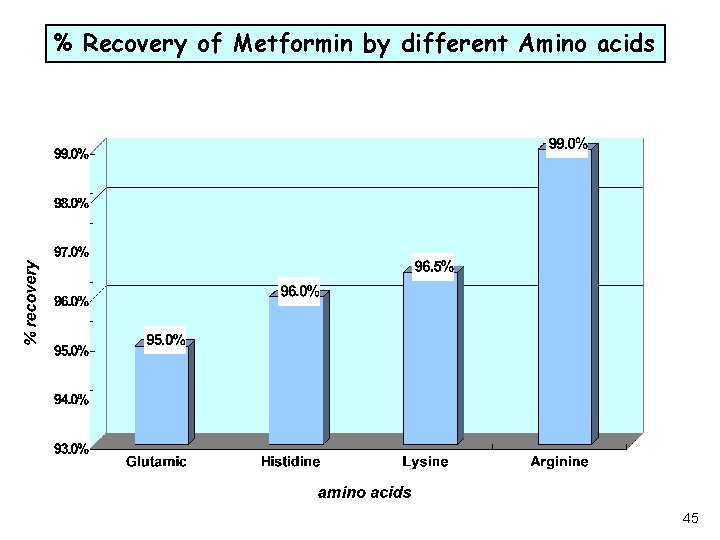
% Recovery of Metformin by different Amino acids 45
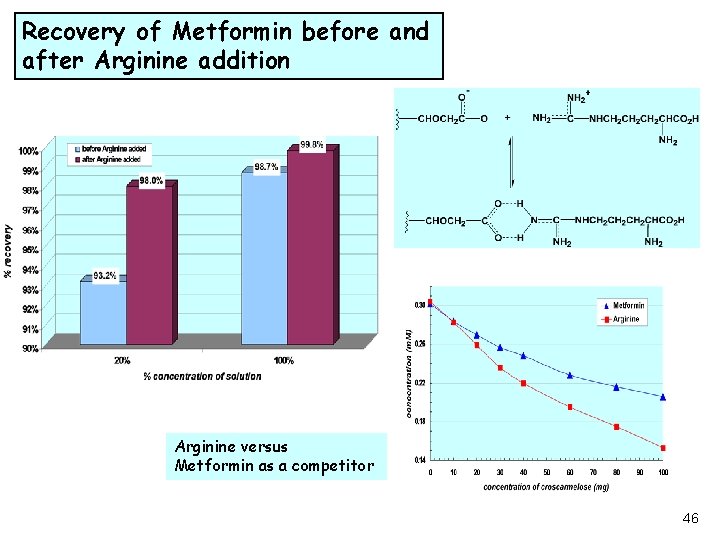
Recovery of Metformin before and after Arginine addition Arginine versus Metformin as a competitor 46
Summary • A strong charge interaction between Metformin and Croscarmelose is formed in aqueous solutions causing sample extraction problem and thus recovery problems. • To eliminate this drug-excipient interaction, Arginine was selected as a competitor versus Metformin. • Even in the lower concentration range Recovery significantly improved. 47
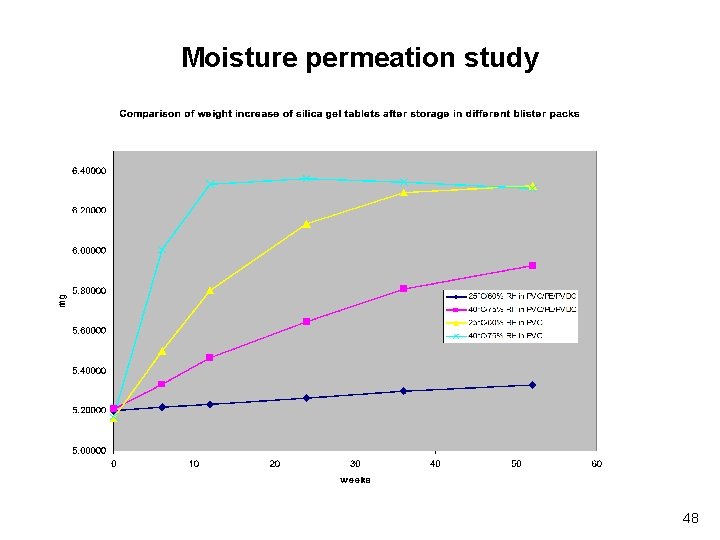
Moisture permeation study 48
- Slides: 48